Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
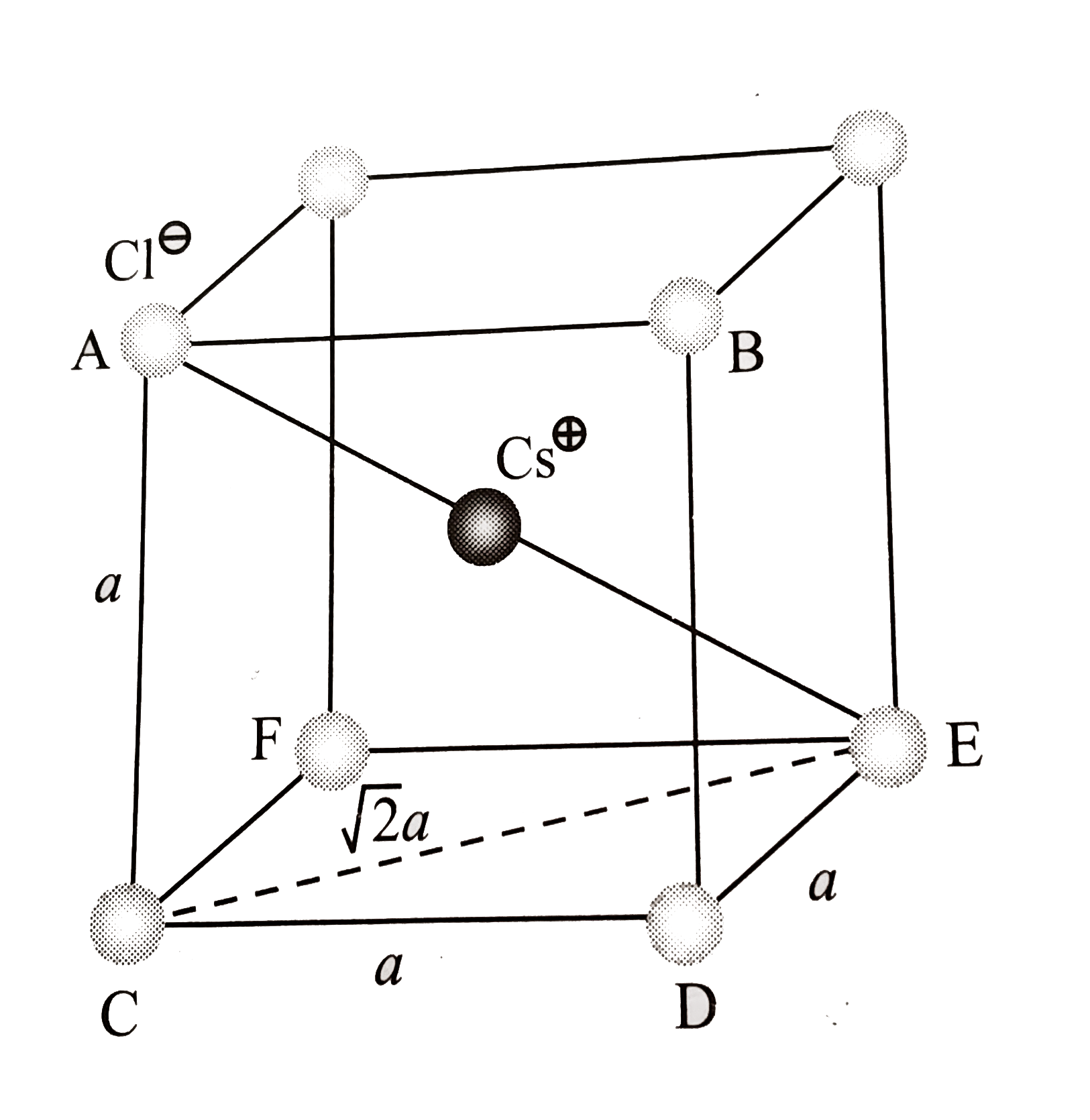
- CsCl has bc c arrangement and its unit cell edge length is 400 pm. Cal...
Text Solution
|
- Which of the following expression is correct in case of a CsCl unit ce...
Text Solution
|
- A solid AB has CsCl-type structure. The edge length of the unit cell i...
Text Solution
|
- A solid AB has CsCl-type structure. The edge length of the unit cell i...
Text Solution
|
- CsCl की संरचना अंतः केन्द्रित घनीय जालक (bcc) की होती है । यदि इसकी क...
Text Solution
|
- CsCl has bcc arrangement. Its unit cell edge length is 400 pm. Its int...
Text Solution
|
- CsCl has bcc arrangement, its unit cell edge length is 400pm, its inte...
Text Solution
|
- Which of the following expressions is correct in case of a CsCl unit c...
Text Solution
|
- CsCl has BCC structure and its unit cell edge length is 400 pm. Determ...
Text Solution
|