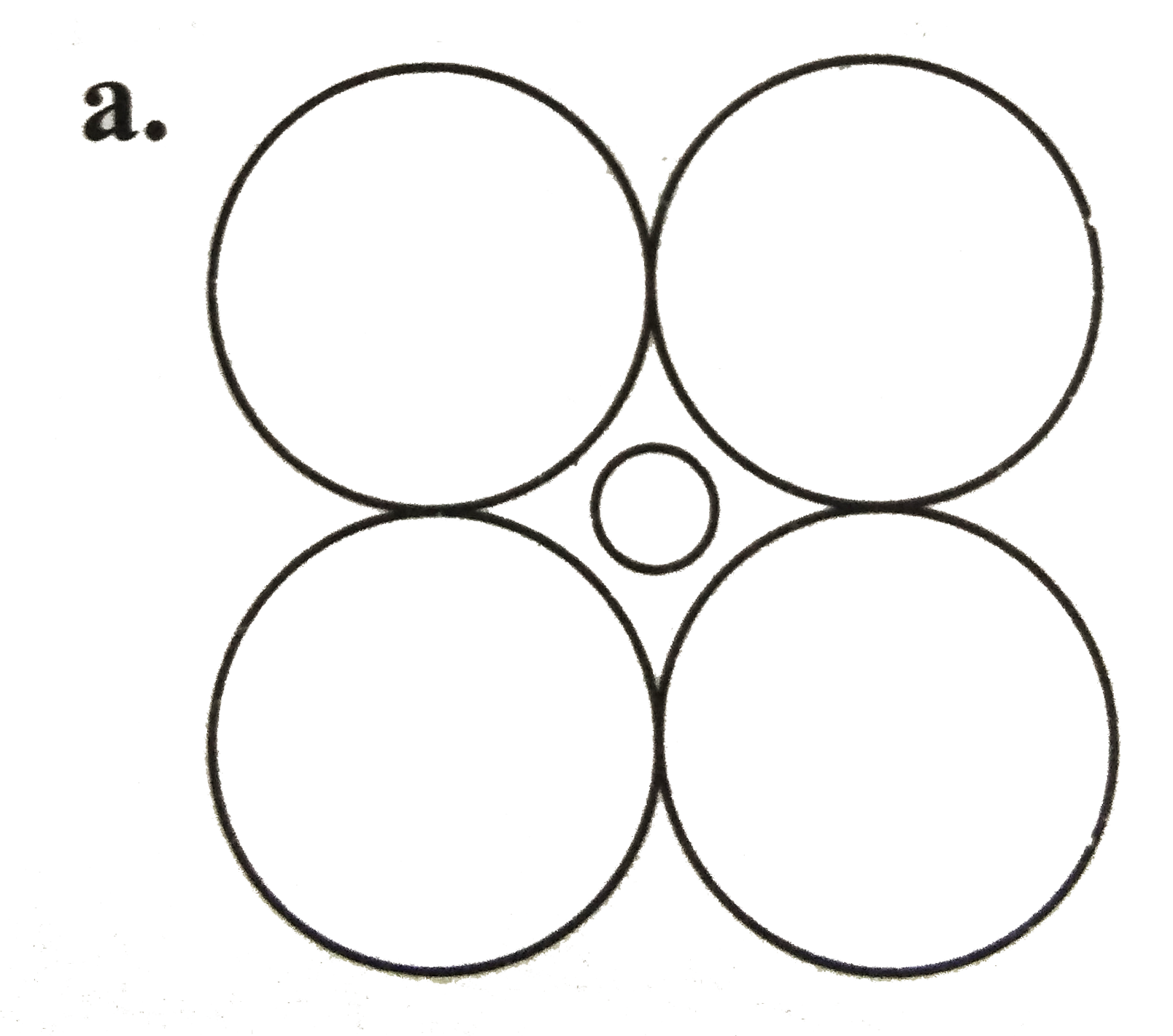
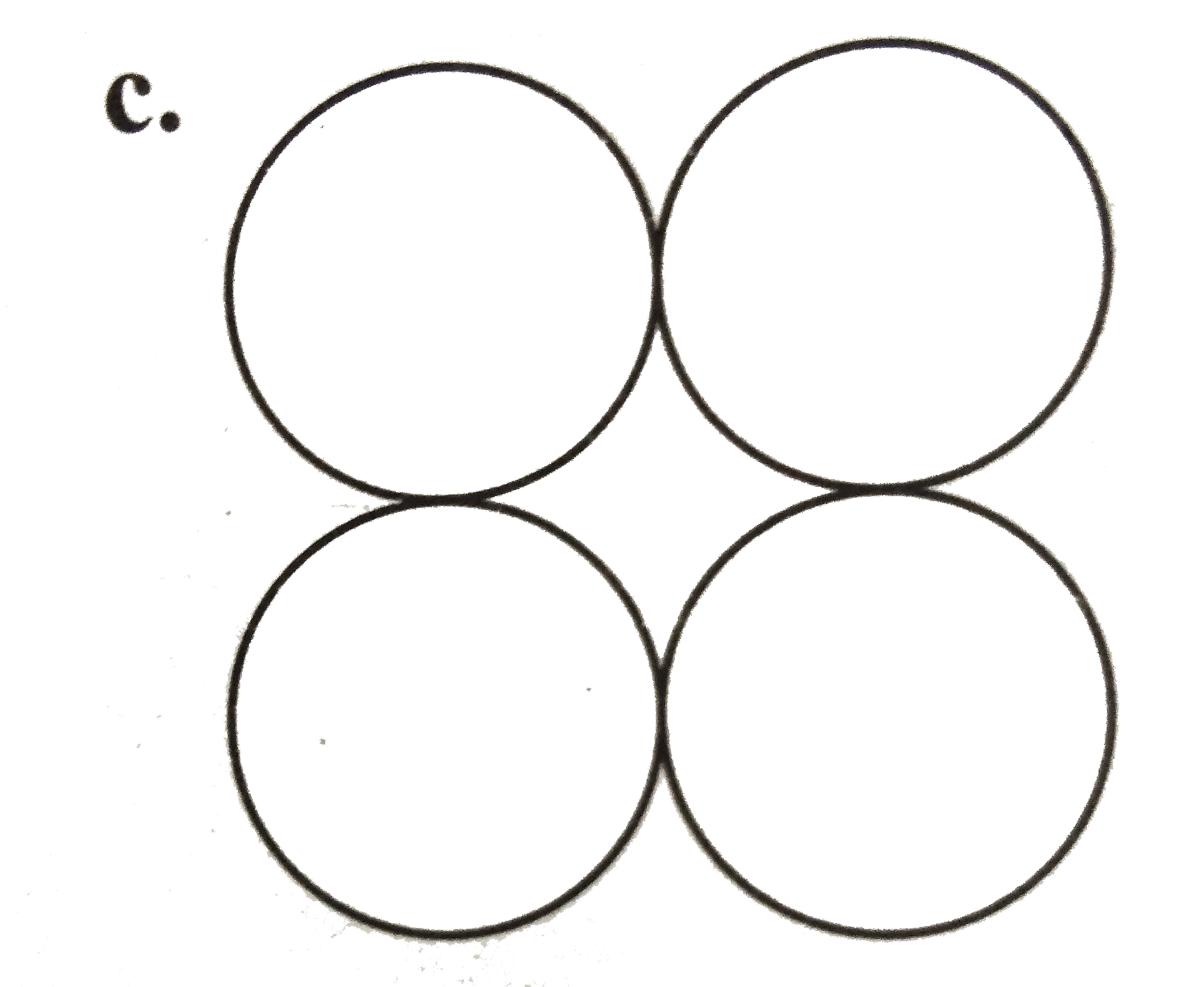
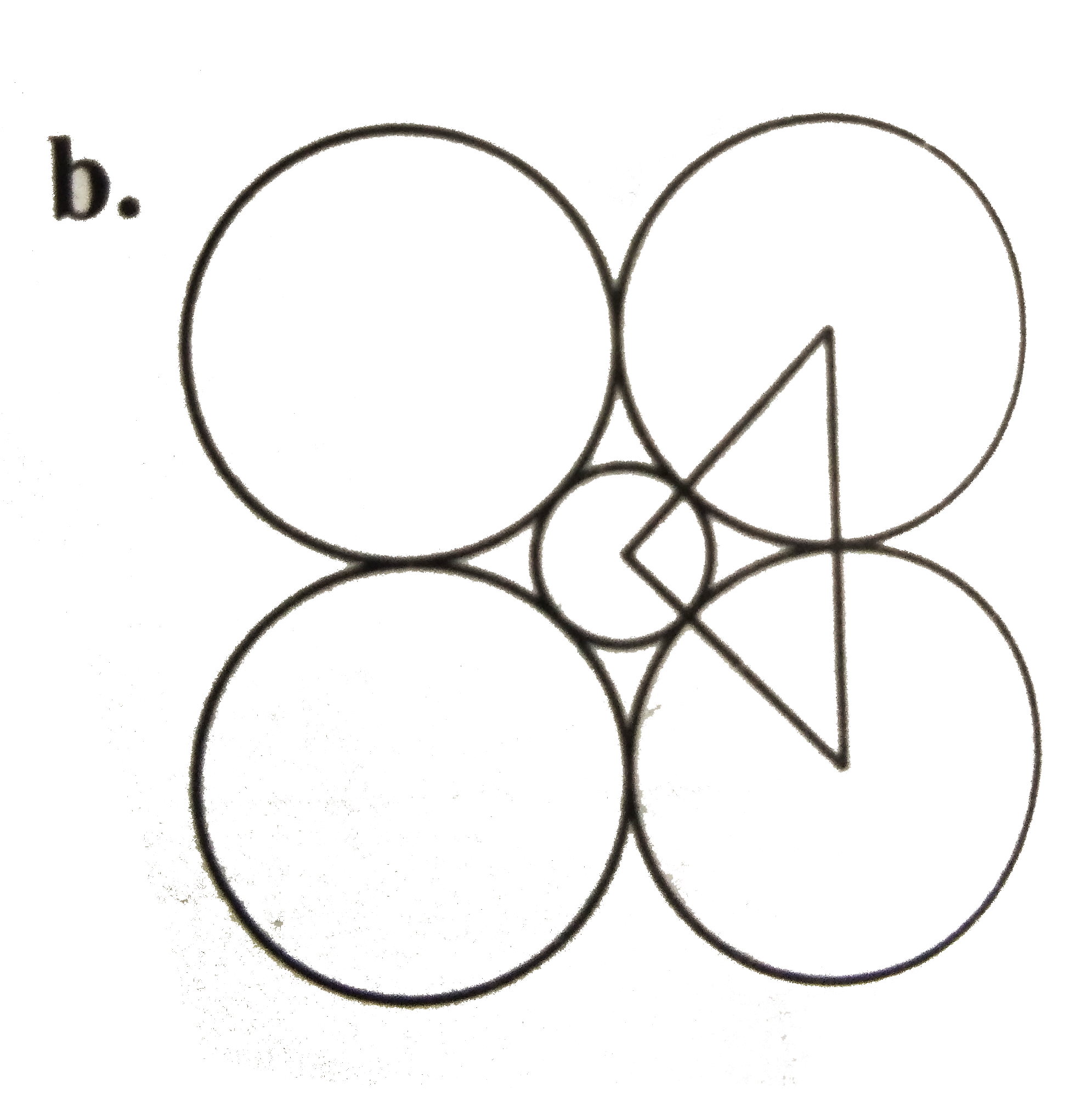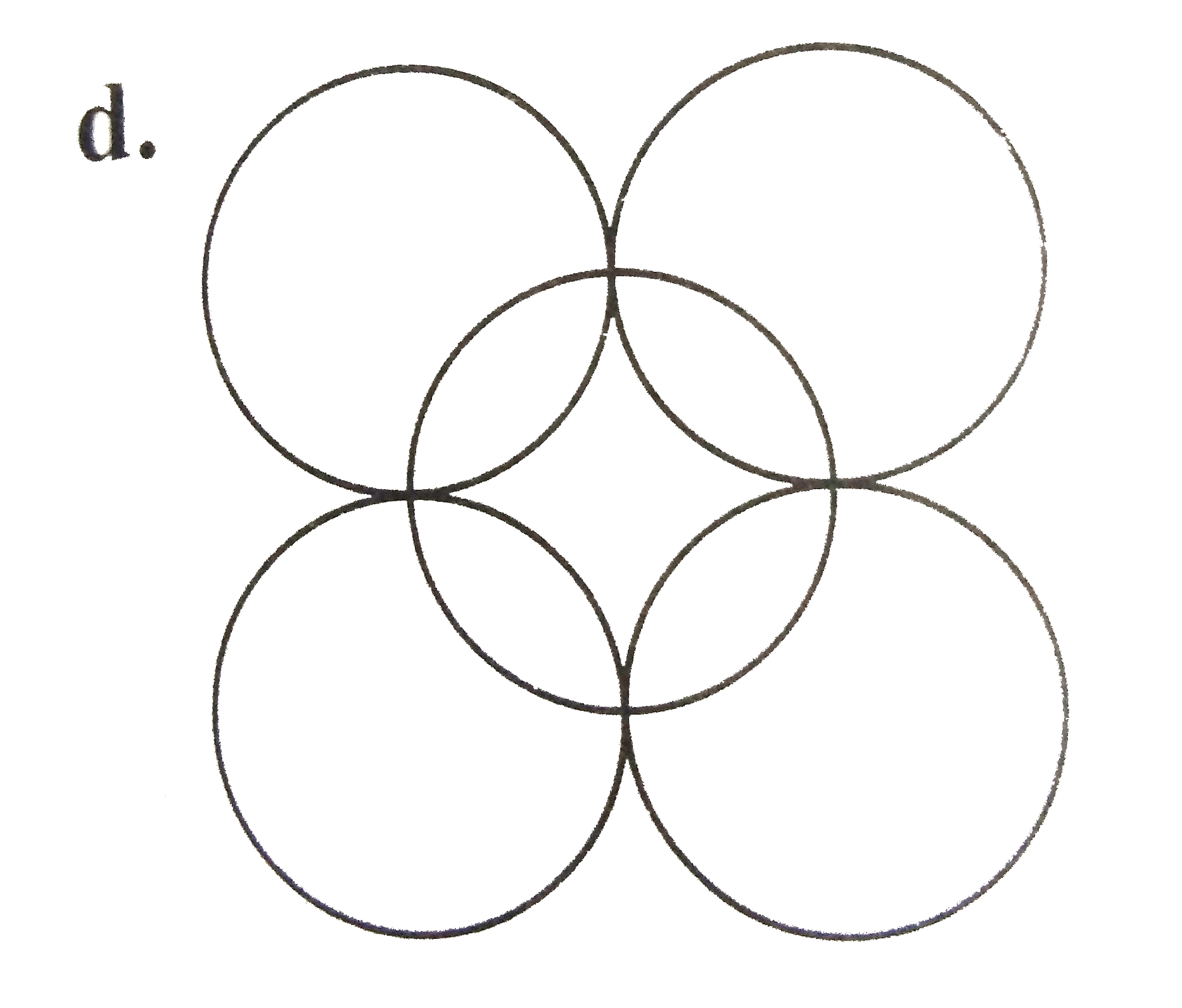A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- AX,AY,BX, and BY have rock salt type structure with following internuc...
Text Solution
|
- AX,AY,BX, and BY have rock salt type structure with following internuc...
Text Solution
|
- AX,AY,BX, and BY have rock salt type structure with following internuc...
Text Solution
|
- AX,AY,BX, and BY have rock salt type structure with following internuc...
Text Solution
|
- AX,AY,BX, and BY have rock salt type structure with following internuc...
Text Solution
|
- Factorize: ax+bx-ay-by
Text Solution
|
- Factorise ax + bx - ay - by
Text Solution
|
- निम्नलिखित के गुणनखंड कीजिये : - ax-by+bx-ay
Text Solution
|
- Factorise : ax + bx – ay – by
Text Solution
|