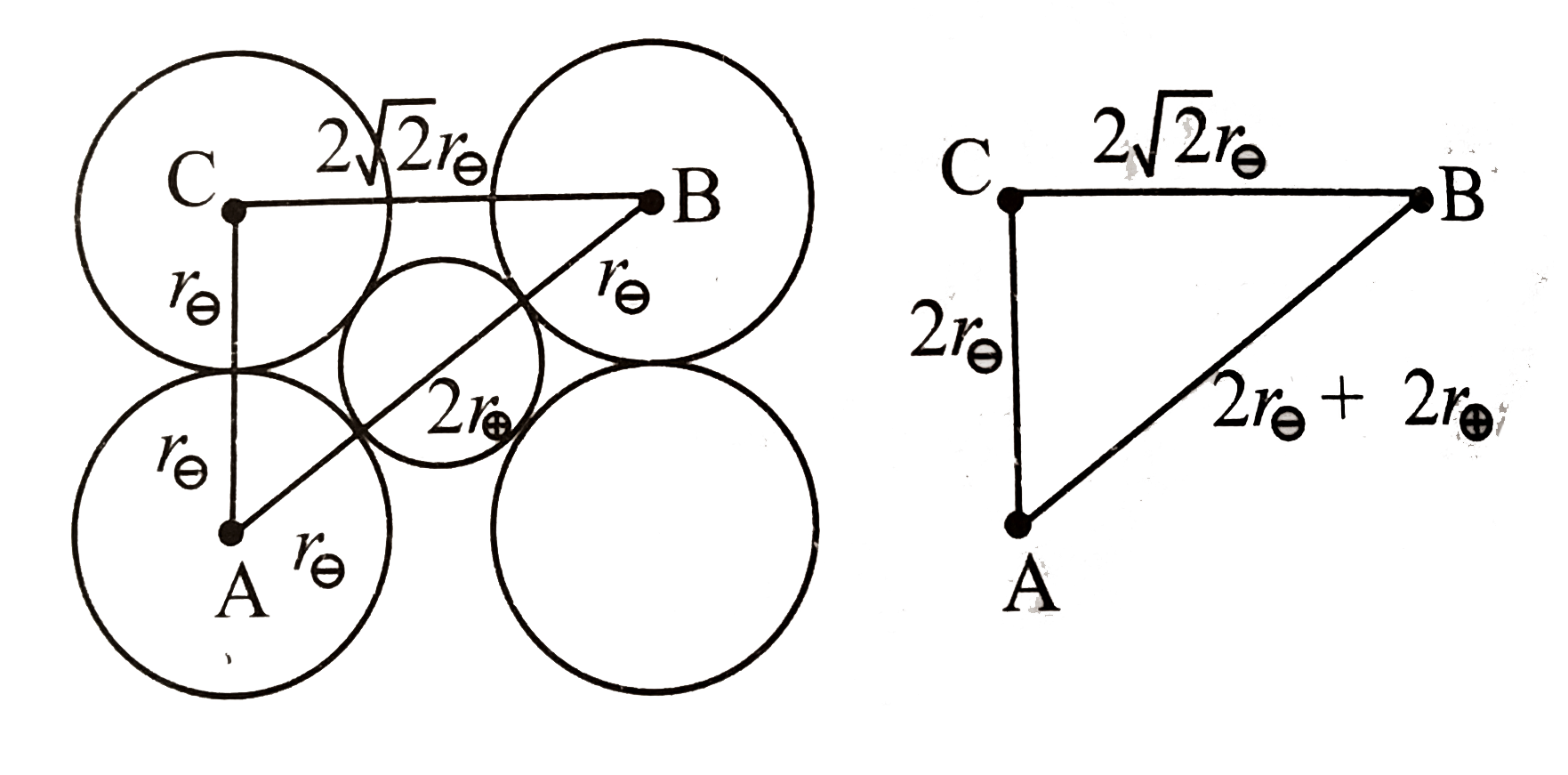A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- AX,AY,BX, and BY have rock salt type structure with following internuc...
Text Solution
|
- AX,AY,BX, and BY have rock salt type structure with following internuc...
Text Solution
|
- AX,AY,BX, and BY have rock salt type structure with following internuc...
Text Solution
|
- AX,AY,BX, and BY have rock salt type structure with following internuc...
Text Solution
|
- AX,AY,BX, and BY have rock salt type structure with following internuc...
Text Solution
|
- A binary solid (AB) has a rock salt structure. If the edge length is 4...
Text Solution
|
- A salt AB cystallises in the CsCl structure. The anions at the corners...
Text Solution
|
- Percentage of void space in AB solid having rock salt structure if (r(...
Text Solution
|
- एक द्विअंगी (A^(+) B^(-)) की संरचना रॉक साल्ट सदृश है । यदि कोर लम्बाई...
Text Solution
|