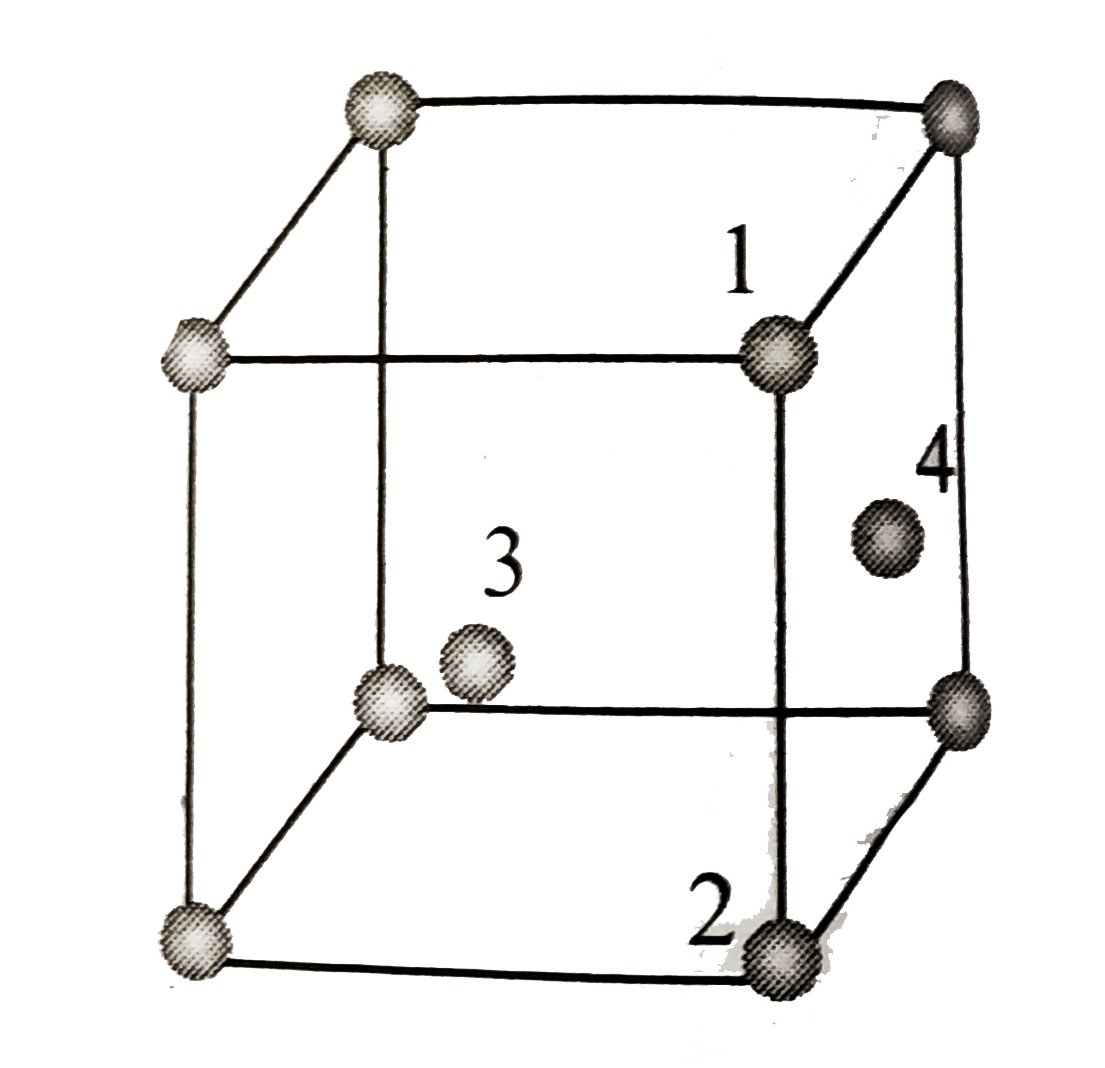
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In fcc unit cell, atoms are numbered as shown below. The atoms not t...
Text Solution
|
- Silver crystallises in a face centred cubic unit cell. Each side of th...
Text Solution
|
- Silver crystallises in a face centred cubic unit cell. Each side of th...
Text Solution
|
- In a face centered unit cell (fcc) the number of atoms present
Text Solution
|
- In a face centred unit cell (fcc), the number of atoms present is :
Text Solution
|
- The number of atoms in a face centred cubic unit cell is:
Text Solution
|
- The number of atoms in a face centred cubic unit cell is:
Text Solution
|
- The number of atoms in a face centred cubic unit cell is:
Text Solution
|
- The number of atoms in a face centred cubic unit cell is:
Text Solution
|