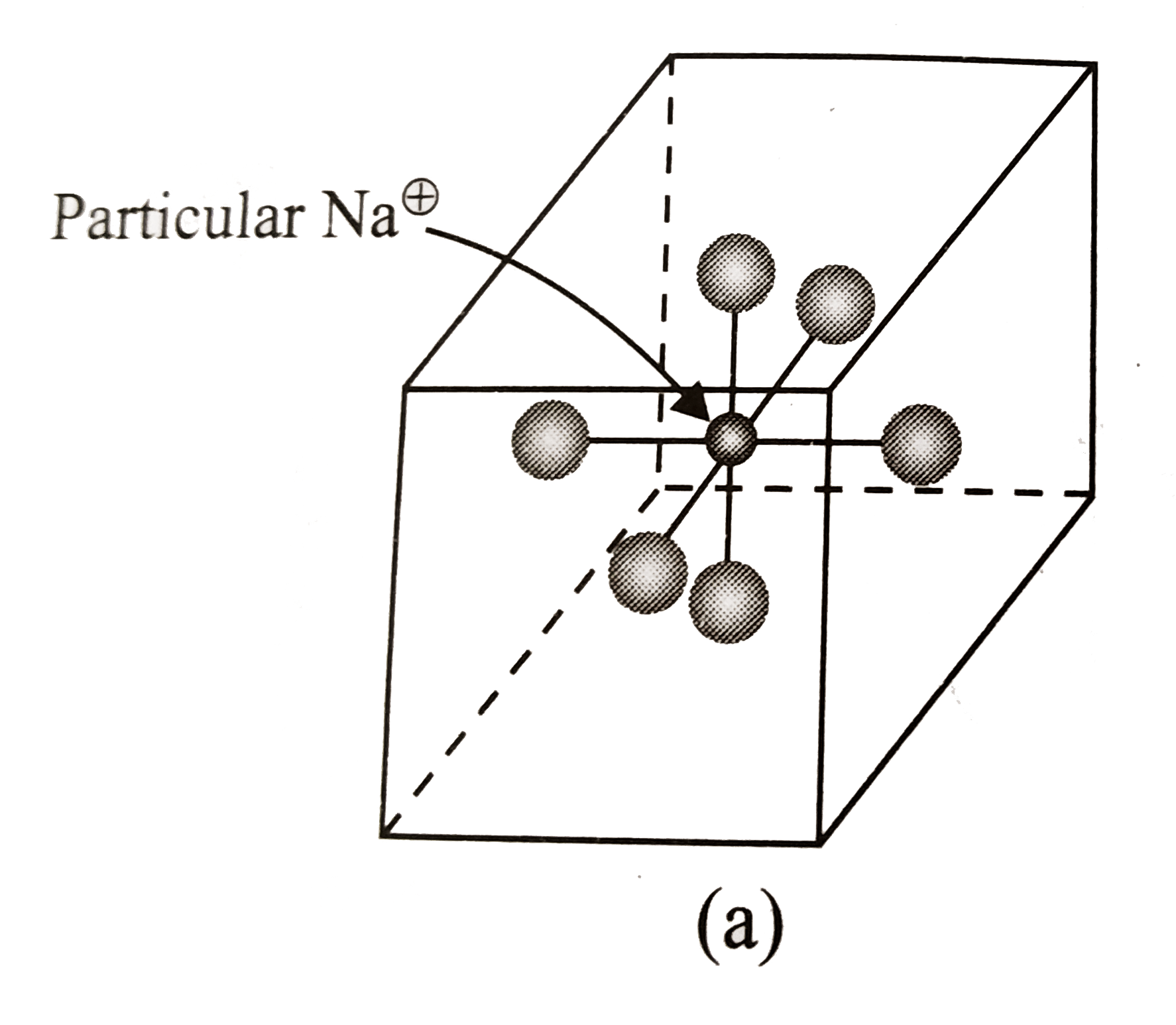
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The number of nearest neighbours and next nearest neighbours of an Na^...
Text Solution
|
- In NaCl ionic crystal, each Na^(+) ion is surrounded by Cl^(-) ions a...
Text Solution
|
- If number of nearest neighbours, next nearest (2nd nearest) neighbour ...
Text Solution
|
- In sodium chloride crystal, the number of next nearst neighbours of ea...
Text Solution
|
- Potassium crystallises in a bcc lattice as shown in figure : (a) Wha...
Text Solution
|
- An element crystallises in a b.c.c. lattice. Nearest and next nearest ...
Text Solution
|
- In the crystal of CsCl ,the nearest neighbours of each Cs ion are :
Text Solution
|
- The number of nearest neighbours and that of next nearest neighbours o...
Text Solution
|
- The number of nearest neighbour and the next neighbour of Na^(+) ion i...
Text Solution
|