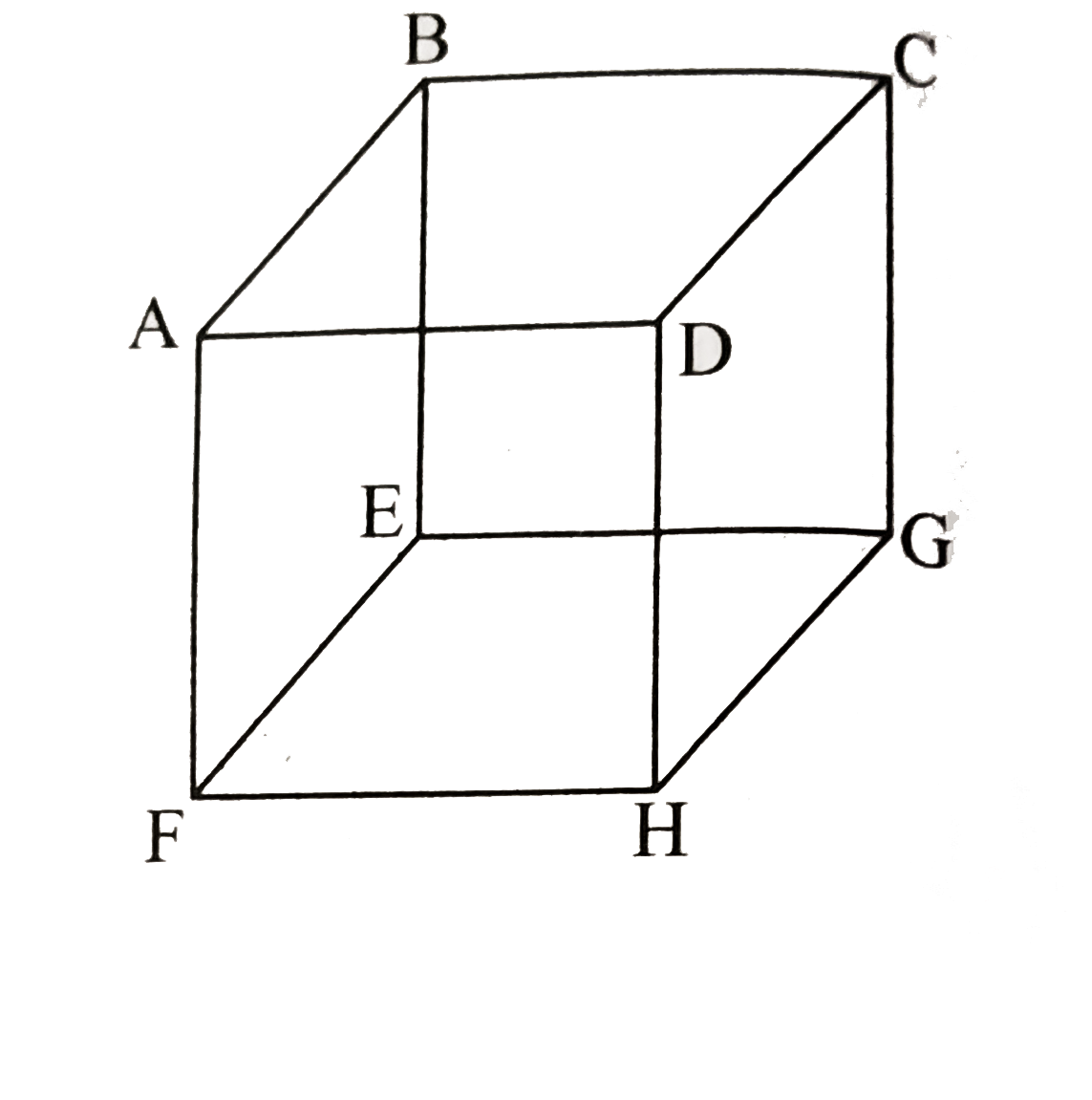
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the cubic lattice given below, the three distances between the atom...
Text Solution
|
- Metallic gold crystallises in face centred cubic lattice with edge-len...
Text Solution
|
- First three nearest neighboure distance for primitive cubic lattice ar...
Text Solution
|
- First three nearestneighbour distances for body centered cubic lattice...
Text Solution
|
- First three nearest neighbout distance for body centrered cubic lattic...
Text Solution
|
- In body-centered cubic lattice given below, the three distances AB, AC...
Text Solution
|
- First three nearest neighboure distance for primitive cubic lattice ...
Text Solution
|
- First three nearest neighbour distance for body centred cubic lattice ...
Text Solution
|
- निम्न में परमाणुओं की संख्या (Z) ज्ञात करो - (a) घनीय (cubic) जालक क...
Text Solution
|