Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
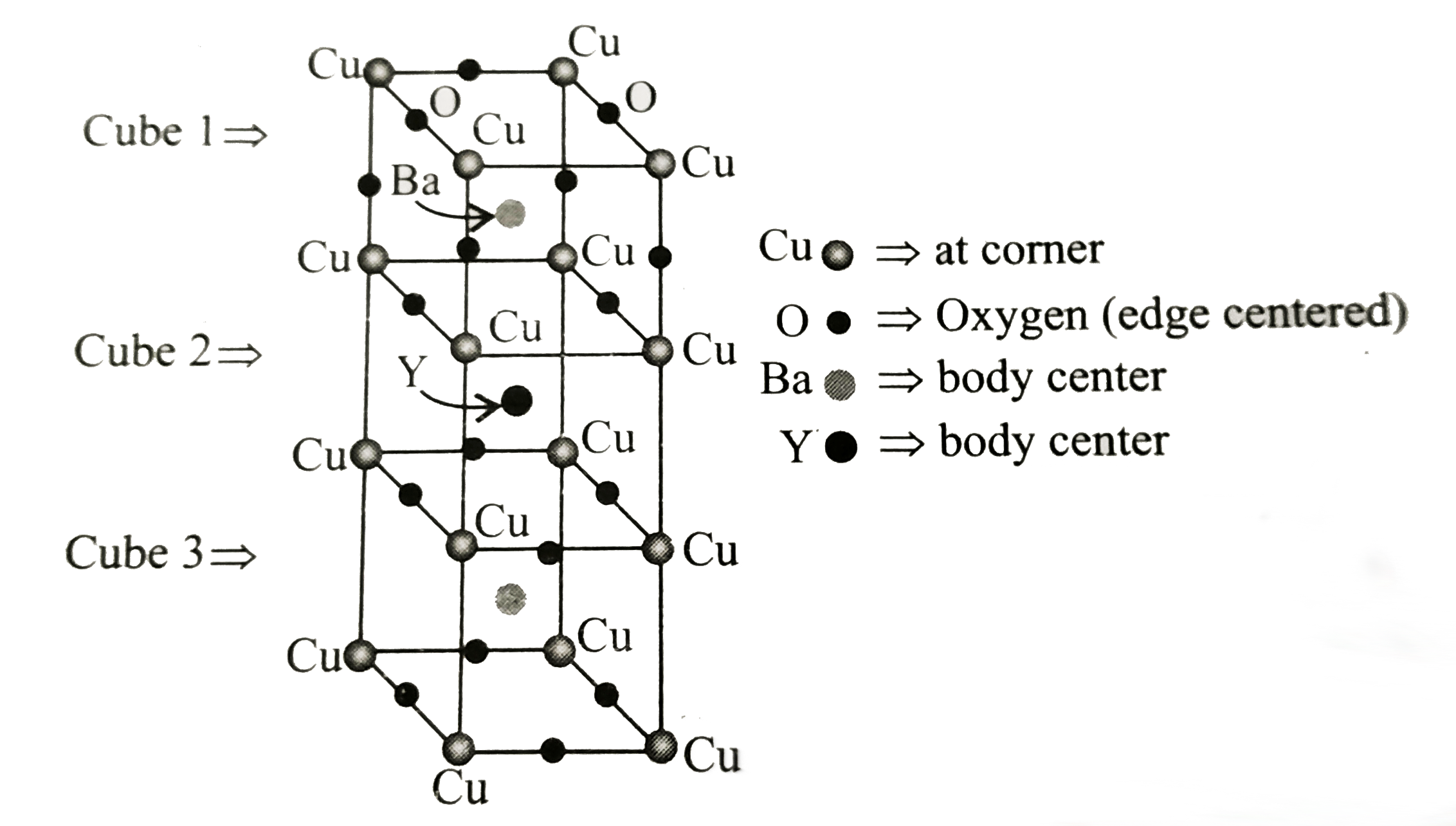
- The following figure shows the unit cell of a compound, i.e., a mixed ...
Text Solution
|
- In the compound Yba(2)Cu(3)O(7) which shows superconductivity, what is...
Text Solution
|
- Structure of a mixed oxide is cubic closed - packed (ccp) .The cubic u...
Text Solution
|
- Structure of a mixed oxide is cubic close packed the cubic unit cell o...
Text Solution
|
- An ether, (A) having molecular formula, C(6)H(14)O, when treated with ...
Text Solution
|
- Structure of a mixed oxide is cubic closed - packed (ccp) .The cubic u...
Text Solution
|
- अतिचालकता दर्शाने वाले यौगिक YBa(2)Cu(3)O(7) में ताँबे की ऑक्सीकरण संख...
Text Solution
|
- (a)Tin stone-Oxides ore (b)Copper pyrite-Oxide ore (c )Zincite-Oxide o...
Text Solution
|
- In the given figure, A B | | D C and A D | | B C show that Δ A B C ...
Text Solution
|