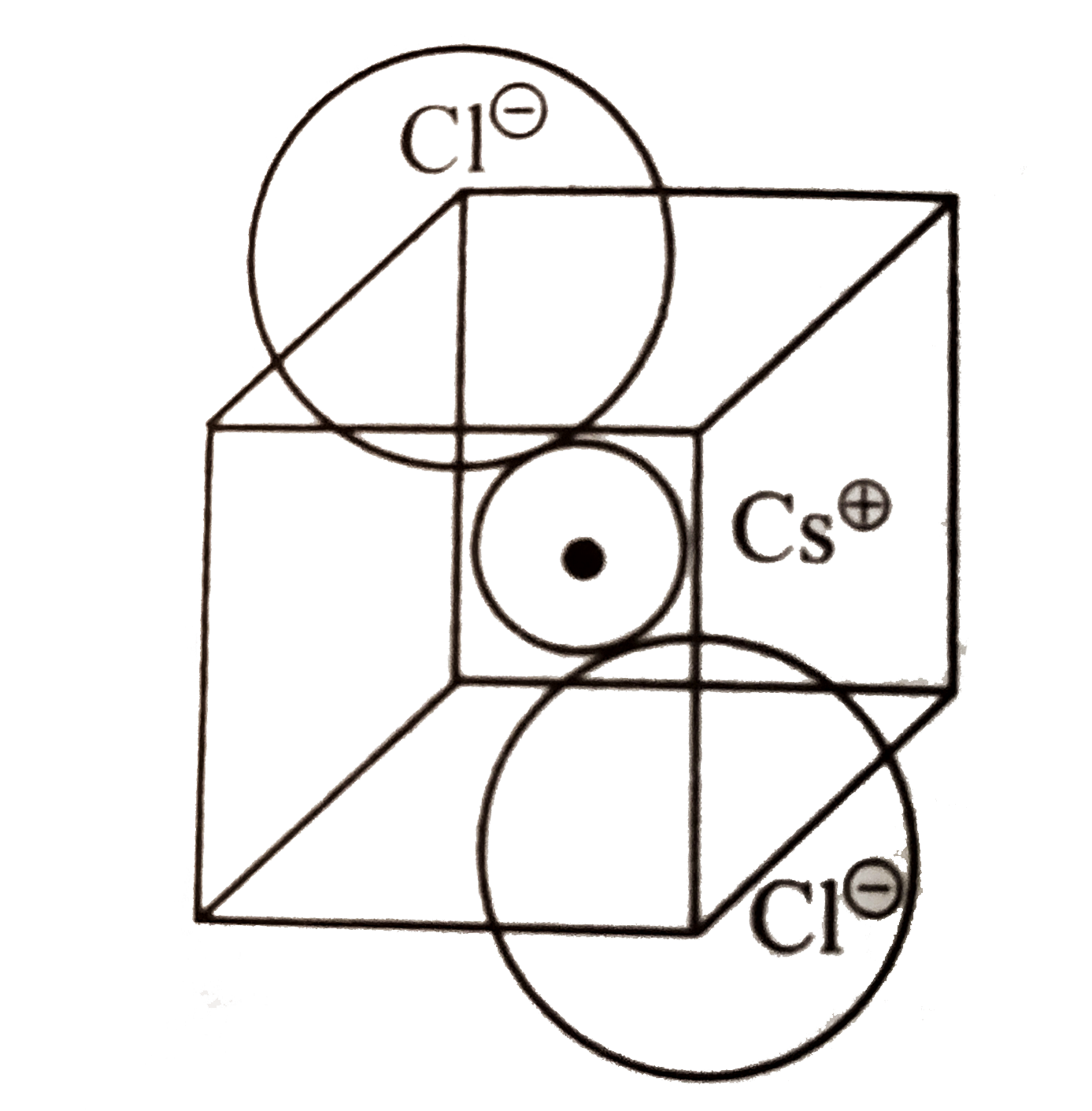
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- CsClcrystallizes in body centred cubic lattice. If 'a' us its edge len...
Text Solution
|
- AB crystallizes in a body centred cubic lattice with edge length a equ...
Text Solution
|
- AB crystallizes in a body centred cubic lattice with edge length 'a' ...
Text Solution
|
- CsCl काय केंद्रित घनाकार जालक में क्रिस्टलीकृत होता है । यदि किनारे की...
Text Solution
|
- AB crystallizes in a body centred cubic lattice with edge length a equ...
Text Solution
|
- Which of the following expressions is correct in case of a CsCl unit c...
Text Solution
|
- AB crystallizes in a body centred cubic lattice with edge length 'a' e...
Text Solution
|
- CsCl crystalises in body centred cubic lattice. If 'a' is its edge len...
Text Solution
|
- AB crystallizes in a body centred cubic lattice with edge length a equ...
Text Solution
|