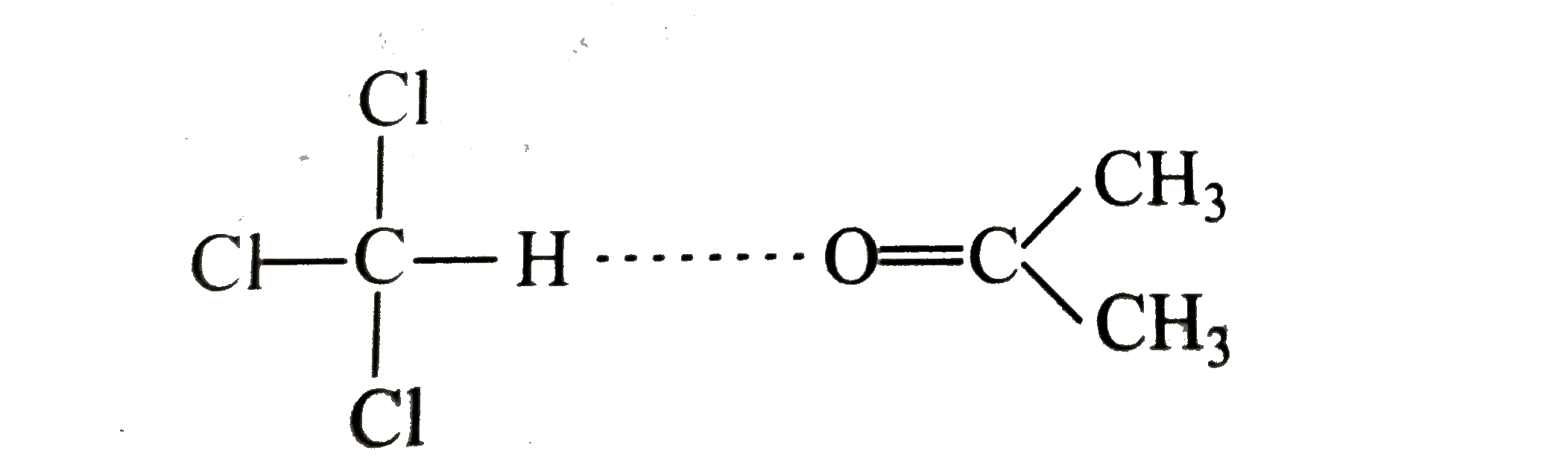
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Give one example each of miscible liquid pairs showing positive and n...
Text Solution
|
- The liquid pair of acetone-chloroform shows a positive deviation form ...
Text Solution
|
- Which liquids pair shows a positive deviation from Raoult's law?
Text Solution
|
- Non-ideal solutions exhibit either positive or negative deviation from...
Text Solution
|
- Which of the following liquid pairs shows a positive deviation from Ra...
Text Solution
|
- Non - ideal solutions exhibit either positive or negative deviations f...
Text Solution
|
- Which of the following liquid pairs shows a positive deviation from Ra...
Text Solution
|
- State Raoult' s law for a soluti.on of 2 volatile liquids. Give an exa...
Text Solution
|
- Which of the following liquid pairs shows a negative deviation from Ra...
Text Solution
|