Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
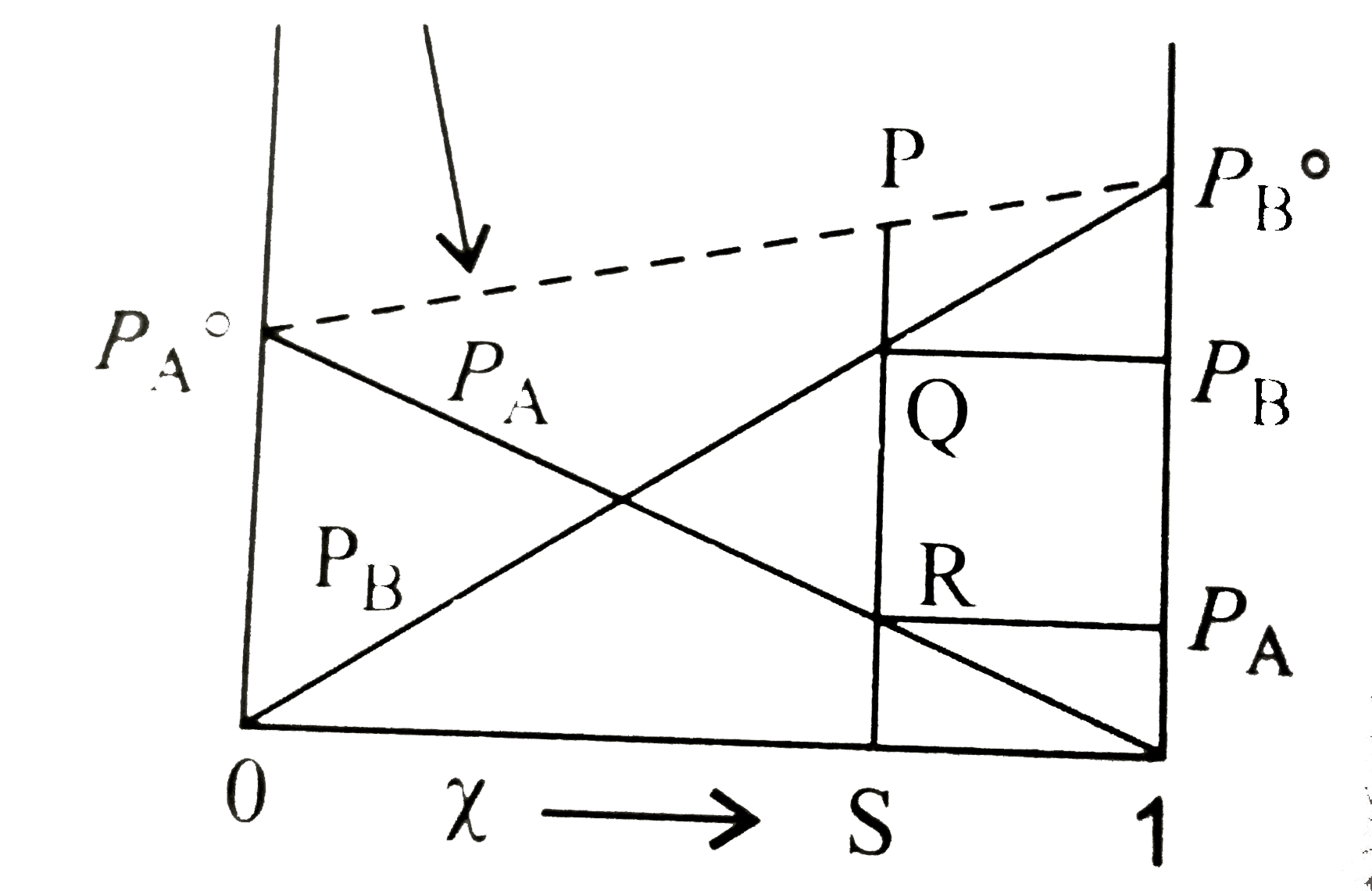
- Consider the follwing vapour pressure composition graph. SP is equal t...
Text Solution
|
- In Figure, P Q > P RdotQ S\ a n d\ R S are the bisectors of L Q and /R...
Text Solution
|
- ((pq)/(qr)-(qr)/(pq))^(3)
Text Solution
|
- In the figure above, PQRS is a trapezium , PQ//SR, QR=RS, and angleQRS...
Text Solution
|
- PQRS एक चतुर्भुज है |जिसके विकर्ण PR तथा QS बिन्दु O पर प्रतिच्छेद करत...
Text Solution
|
- A, B, C, D are the mid-points of the sides PQ, QR, RS and SP respectiv...
Text Solution
|
- In the given figure, PQRS is a trapezium in which PQ || SR And PS = QR...
Text Solution
|
- P,Q,R and S are the points in a vertical line. It is given that PQ=QR=...
Text Solution
|
- If A = [{:(-qr , p(q + r) , pr + pq),(pq + qr, -pr, pq + qr ),(qr + pr...
Text Solution
|