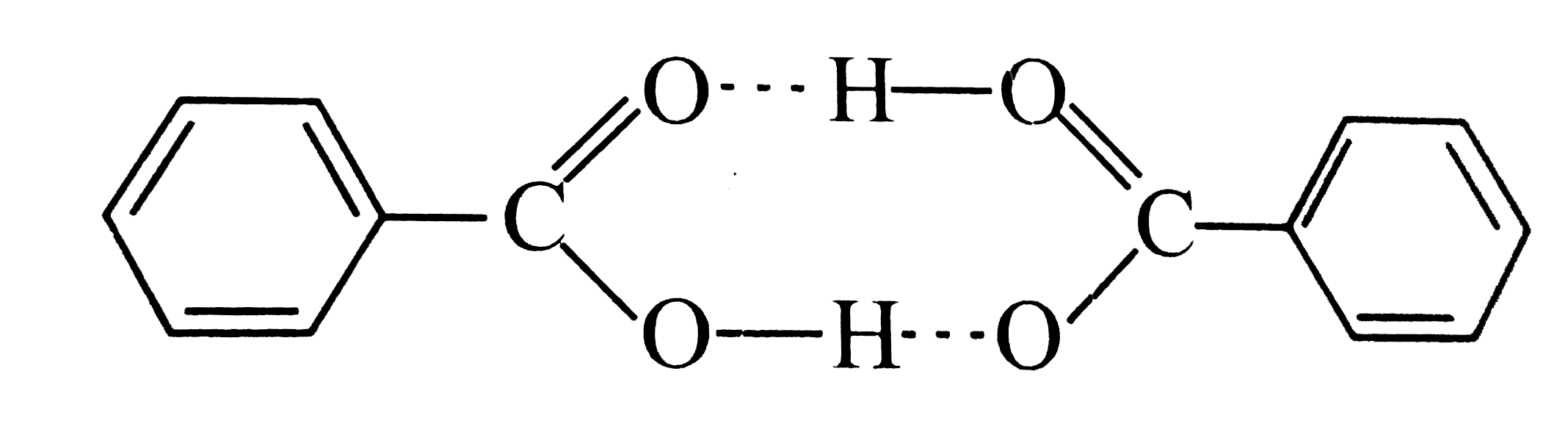
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- 12.2 g of benzoic acid is dissolved in (i) 1 kg acetone (K(b) = 1.9 K ...
Text Solution
|
- 12.2 g of benzoic acid (Mw=122) in 100 g benzene has depression in fre...
Text Solution
|
- 12.2 g of benzoic acid (Mw=122) in 100 g water has elevation in boilin...
Text Solution
|
- 1.22 g of benzoic acid is dissolved in acetone and benzene separately....
Text Solution
|
- 1.22 g of benzoic acid is dissolved in (i) 100g acetone (K(b) for acet...
Text Solution
|
- 1.22 g of benzoic acid is dissolved in 100 g of acetone and 100 g of a...
Text Solution
|
- 3.9 g of benzoic acid dissoved in 49 g of benzene shows a depression i...
Text Solution
|
- 3.9 g of benzoic acid dissolved in 49 g of benzene shows a depression ...
Text Solution
|
- 0.3 ग्राम बैन्जोइक अम्ल 20 ग्राम बैंजीन में घुला हुआ है। इस विलयन का ...
Text Solution
|