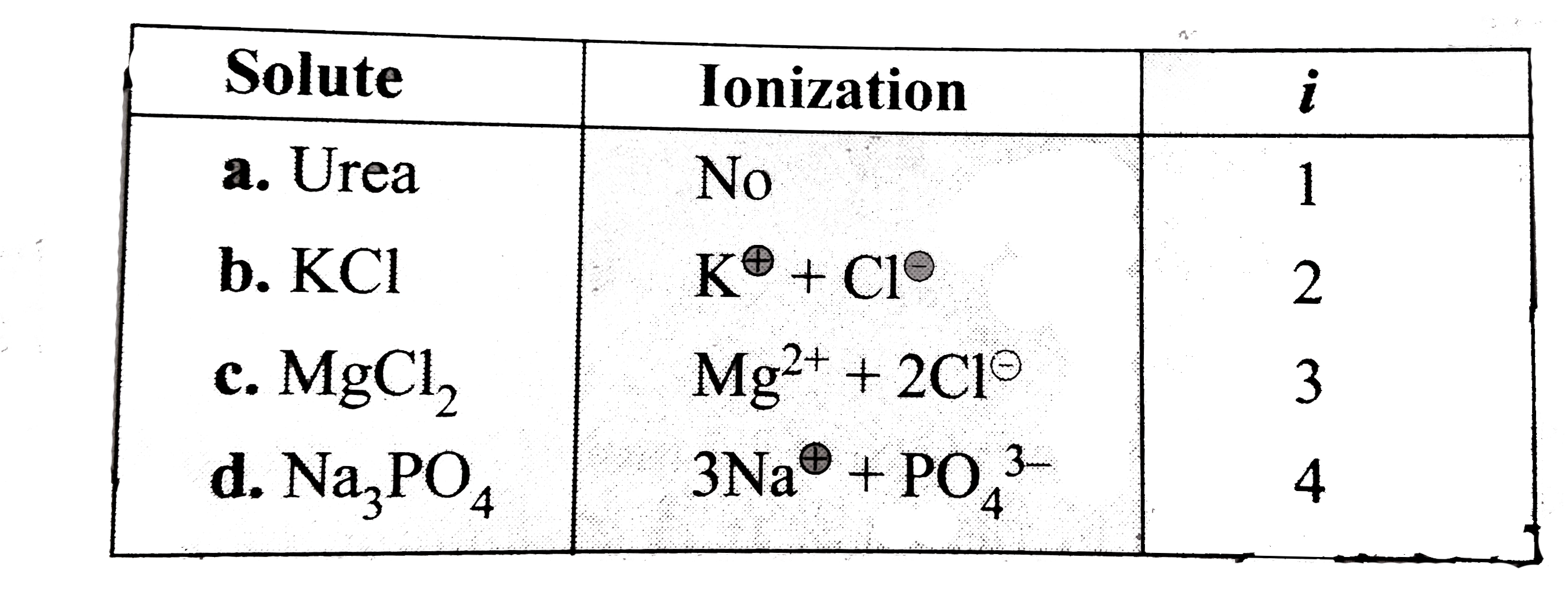Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Follwing are equimolal aqueous solution: a.1 m urea , b.1 m KCl , c....
Text Solution
|
- Arrange the following solutions as directed: Increasing order of boi...
Text Solution
|
- Following are equimolal ( = f equimolar) aqueous solutions (A) 1 m glu...
Text Solution
|
- An aqueous solution of 0.01 M KCl cause the same elevation in boiling ...
Text Solution
|
- Following are equimolal (=equimolar) aqueous solutions. (A) 1 m glucos...
Text Solution
|
- निम्नलिखित विलयनों को कारण सहित निर्देशानुसार व्यवस्थित कीजिए - (i)...
Text Solution
|
- निम्नलिखित विलयनों में किसका सर्वाधिक परासरण दाब है ? (i) 1 M KCl , ...
Text Solution
|
- निन्नलिखित विलयनों में से किसका परासरण दाब सर्वाधिक है ? (i) 1 M NaC...
Text Solution
|
- Arrange the following aqueous solutions in the order of their increasi...
Text Solution
|