Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
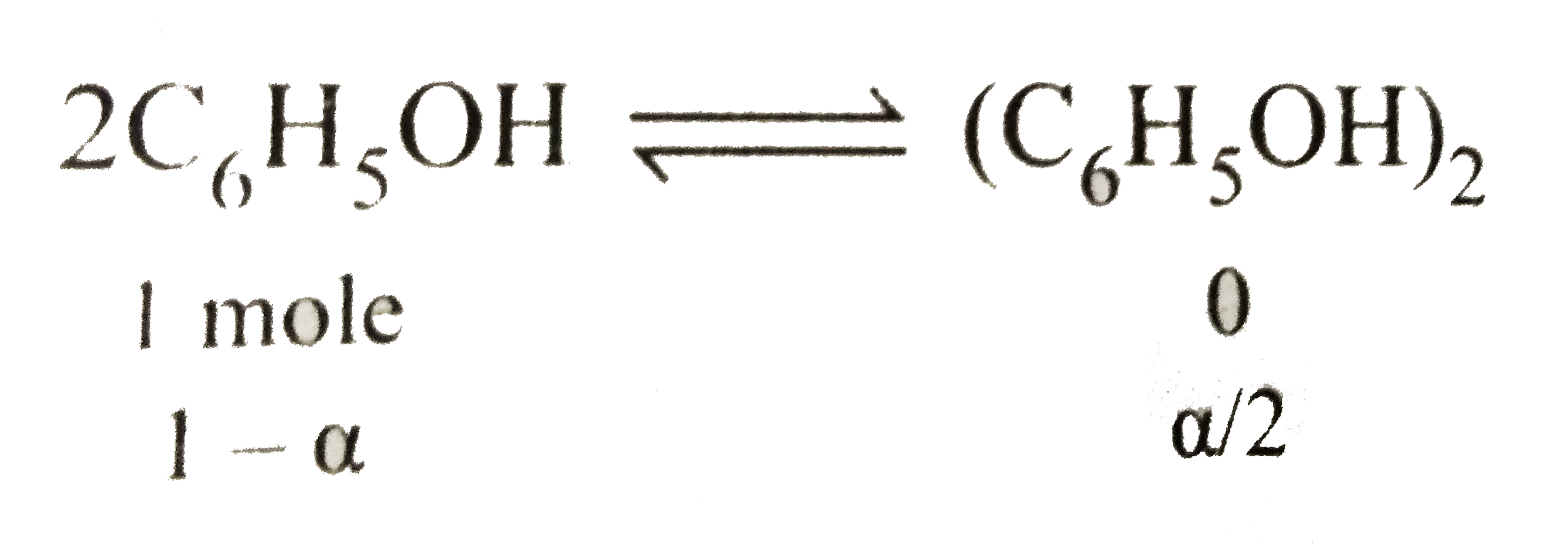
- Phenol associates in benzene to certain extent to form a dimer. A solu...
Text Solution
|
- The fraction of phenol dimerised in benzene if 20 g of phenol in 1 kg ...
Text Solution
|
- Phenol associates in benzene to a certain extent to form a dimer. A so...
Text Solution
|
- The fraction of phenol dimerised in benzene if 20 g of phenol in 1 ...
Text Solution
|
- phenol associates in benzene to a certain extent in dimerisation react...
Text Solution
|
- Phenol associaes in bensen to a certain extent to from a dimer. A solu...
Text Solution
|
- Phenol associates in benzene to a certain extent to form dimer. A solu...
Text Solution
|
- Phenol associates in Benzene to a certain extent to form dimer. A solu...
Text Solution
|
- फीनोल को बेंजीन में घोलने पर उसके दो-दो अणु संगुणन होकर एक बड़ा अणु बना...
Text Solution
|