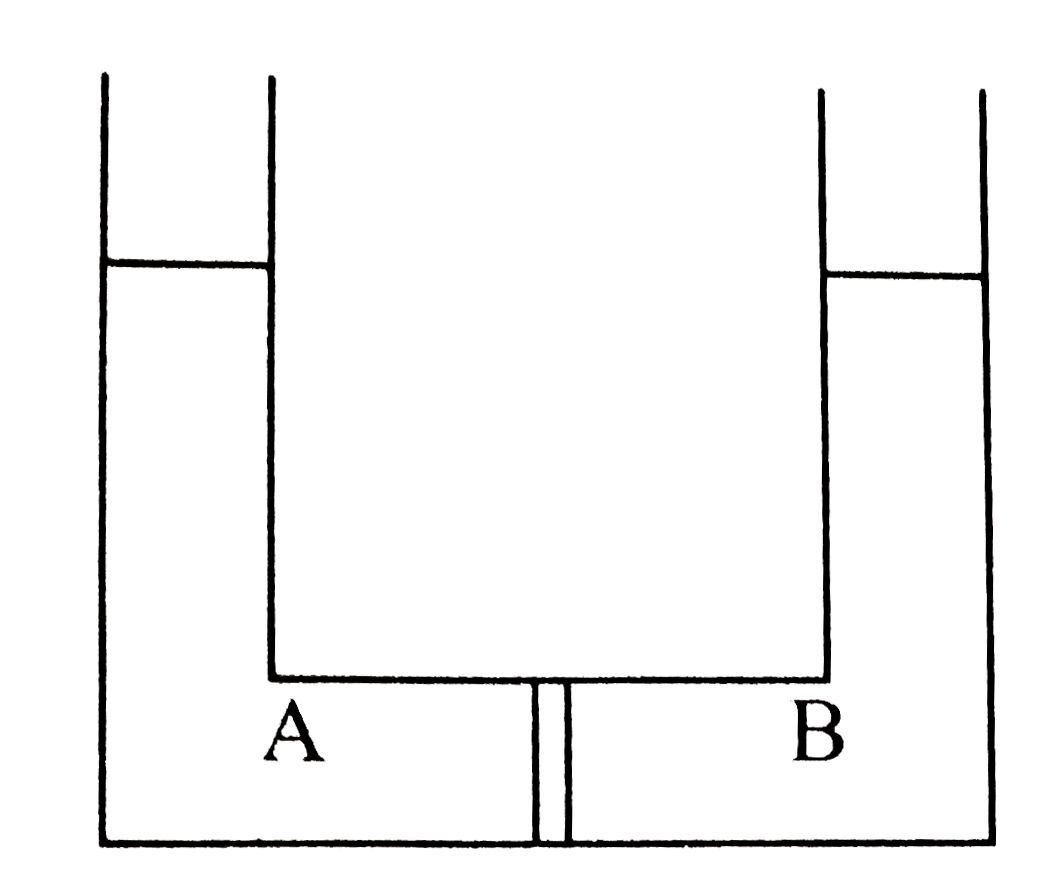
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Compartment A and B have the following combinations of solution: {:(...
Text Solution
|
- Compartment A and B have the following combinations of solution: {:(...
Text Solution
|
- Compartment A and B have the following combinations of solution: {:(...
Text Solution
|
- Compartment A and B have the following combinations of solution: {:(...
Text Solution
|
- Compartment A and B have the following combinations of solution: {:(...
Text Solution
|
- कथन समान ताप पर 1 M NaCl विलयन का परासरण दाब 1 M ग्लूकोज विलयन से उ...
Text Solution
|
- Which of the following solutions have highest boiling point and why ? ...
Text Solution
|
- Arrange the following solutions in the decreasing order of specific co...
Text Solution
|
- একটি নির্দিষ্ট উষ্ণতায় 0.05(M) NaCl-দ্রবন, একটি গ্লুকজ দ্রবনের সঙ্গে ...
Text Solution
|