A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
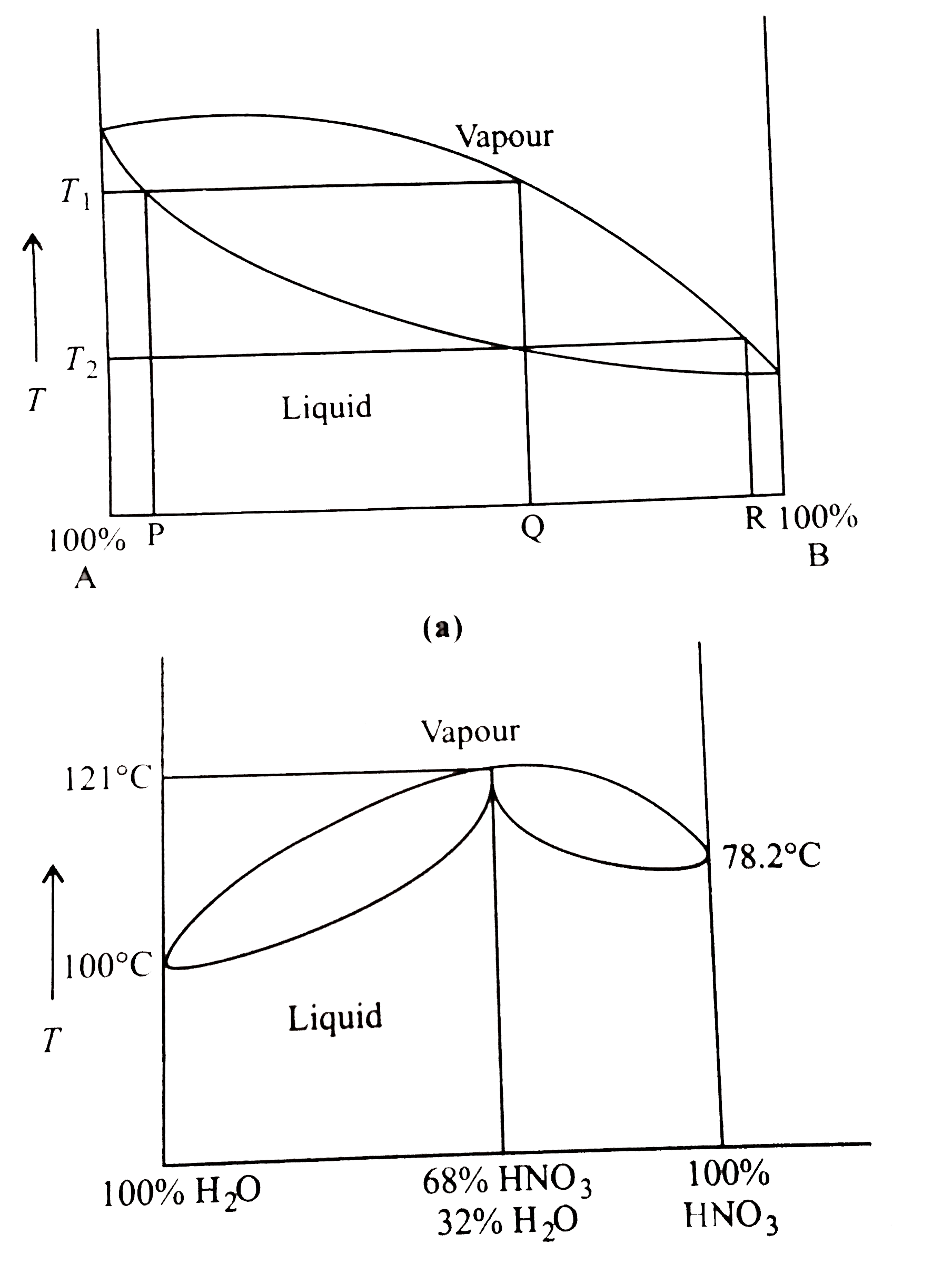
- represents the distillation of mixture of liquid A and liquid B which ...
Text Solution
|
- represents the distillation of mixture of liquid A and liquid B which ...
Text Solution
|
- represents the distillation of mixture of liquid A and liquid B which ...
Text Solution
|
- represents the distillation of mixture of liquid A and liquid B which ...
Text Solution
|
- represents the distillation of mixture of liquid A and liquid B which ...
Text Solution
|
- represents the distillation of mixture of liquid A and liquid B which ...
Text Solution
|
- Pure water boils at 373.15 K and nitric acid boils at 359.15 K . An az...
Text Solution
|
- liquids can be separated from their mixtures by the method of distilat...
Text Solution
|
- दो द्रवों का स्थिर क्वथनांकी (azeotropic) मिश्रण दोनों शुद्ध द्रवों के...
Text Solution
|