represents the distillation of mixture of liquid `A` and liquid `B` which gives both of pure liquid `A` and `B` . Represents the azeotropic mixture of `HNO_(3)` and `H_(2)O` which distillation gives an azeotropic mixture and either of pure liquid. We cannot separate both the pure liquid, i.e., `H_(2)O` and `HNO_(3)`.
a solution of `50%` of `B` on distillation results into
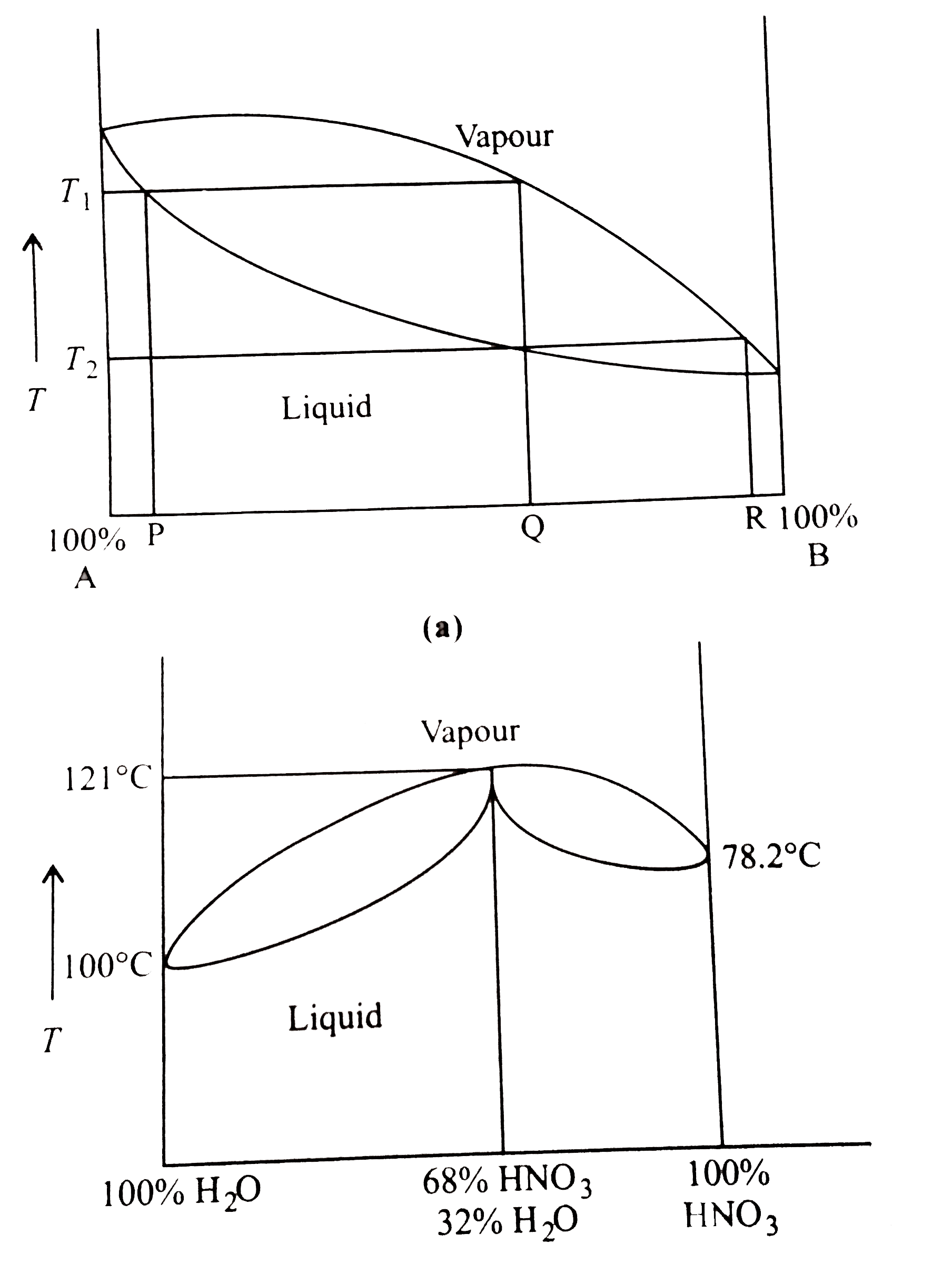
represents the distillation of mixture of liquid `A` and liquid `B` which gives both of pure liquid `A` and `B` . Represents the azeotropic mixture of `HNO_(3)` and `H_(2)O` which distillation gives an azeotropic mixture and either of pure liquid. We cannot separate both the pure liquid, i.e., `H_(2)O` and `HNO_(3)`.
a solution of `50%` of `B` on distillation results into

a solution of `50%` of `B` on distillation results into

A
Separation of an azeotropic mixture and pure `A`.
B
Separation of an azeotropic mixture and pure `B`.
C
Separation of both pure `A` and pure `B`.
D
None of these
Text Solution
Verified by Experts
The correct Answer is:
C
`A` and `B` do not form any azeotropic mixture, therefore they can be easily separable.
Similar Questions
Explore conceptually related problems
A mixture of two immiscible liquids A and B , having vapour pressure in pure state obeys the following relationship if chi_(A) and chi_(B) are mole fractions of A and B in vapour phase over the solution
The vapour pressure of two liquids A and B in their pure states are in ratio of 1:2 . A binary solution of A and B contains A and B in the mole proportion of 1:2 . The mole fraction of A in the vapour phase of the solution will be
A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vapourizes to form a more disordered gas. When a solute is present, there is additional contribution to the entropy of the liquid due to increased randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas. Thus, a solute (non-volatile) lowers the vapour pressure of a liquid, and hence a higher boiling point of the solution. Similarly, the greater randomness of the solution opposes the tendercy to freeze. In consequence, a lower temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution. The elevation in boiling point (DeltaT_(b)) and depression in freezing point (DeltaT_(f)) of a solution are the colligative properties which depend only on the concentration of particles of the solute and not their identity. For dilute solutions, (DeltaT_(b)) and (DeltaT_(f)) are proportional to the molarity of the solute in the solution. A mixture of two immiscible liquids at a constant pressure of 1.0 atm boils at temperature
A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vapourizes to form a more disordered gas. When a solute is present, there is additional contribution to the entropy of the liquid due to increased randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas. Thus, a solute (non-volatile) lowers the vapour pressure of a liquid, and hence a higher boiling point of the solution. Similarly, the greater randomness of the solution opposes the tendercy to freeze. In consequence, a lower temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution. The elevation in boiling point (DeltaT_(b)) and depression in freezing point (DeltaT_(f)) of a solution are the colligative properties which depend only on the concentration of particles of the solute and not their identity. For dilute solutions, (DeltaT_(b)) and (DeltaT_(f)) are proportional to the molarity of the solute in the solution. A liquid possessing which of the following characteristics will be most suitable for determining the molecular mass of a compound by cryoscopic measurements?
A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vapourizes to form a more disordered gas. When a solute is present, there is additional contribution to the entropy of the liquid due to increased randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas. Thus, a solute (non-volatile) lowers the vapour pressure of a liquid, and hence a higher boiling point of the solution. Similarly, the greater randomness of the solution opposes the tendercy to freeze. In consequence, a lower temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution. The elevation in boiling point (DeltaT_(b)) and depression in freezing point (DeltaT_(f)) of a solution are the colligative properties which depend only on the concentration of particles of the solute and not their identity. For dilute solutions, (DeltaT_(b)) and (DeltaT_(f)) are proportional to the molarity of the solute in the solution. Dissolution of a non-volatile solute into a liquid leads to
A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vapourizes to form a more disordered gas. When a solute is present, there is additional contribution to the entropy of the liquid due to increased randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas. Thus, a solute (non-volatile) lowers the vapour pressure of a liquid, and hence a higher boiling point of the solution. Similarly, the greater randomness of the solution opposes the tendercy to freeze. In consequence, a lower temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution. The elevation in boiling point (DeltaT_(b)) and depression in freezing point (DeltaT_(f)) of a solution are the colligative properties which depend only on the concentration of particles of the solute and not their identity. For dilute solutions, (DeltaT_(b)) and (DeltaT_(f)) are proportional to the molarity of the solute in the solution. To aqueous solution of Nal , increasing amounts of solid Hgl_(2) is added. The vapour pressure of the solution
The vapour pressures of two liquids A and B in their pure states are in the ratio of 1 : 2 . A binary solution of A and B contains A and B in the mole proportion of 1: 2 . The mole fraction of A in the vapour phase of the solution will be
Two miscible liquids A and B having vapour pressure in pure state P_(A)^(@) and P_(B)^(@) are mixed in mole fraction chi_(A) and chi_(B) to get a mixtue having total vapour vapour pressure of mixture P_(M) . Which of the following relations are correct?
Recommended Questions
- represents the distillation of mixture of liquid A and liquid B which ...
Text Solution
|
- represents the distillation of mixture of liquid A and liquid B which ...
Text Solution
|
- represents the distillation of mixture of liquid A and liquid B which ...
Text Solution
|
- represents the distillation of mixture of liquid A and liquid B which ...
Text Solution
|
- represents the distillation of mixture of liquid A and liquid B which ...
Text Solution
|
- represents the distillation of mixture of liquid A and liquid B which ...
Text Solution
|
- Pure water boils at 373.15 K and nitric acid boils at 359.15 K . An az...
Text Solution
|
- दो द्रवों का स्थिर क्वथनांकी (azeotropic) मिश्रण दोनों शुद्ध द्रवों के...
Text Solution
|
- शुद्ध द्रव के समान स्थिर ताप पर उबलने तथा संघटन में बिना के आसवित होन...
Text Solution
|