A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
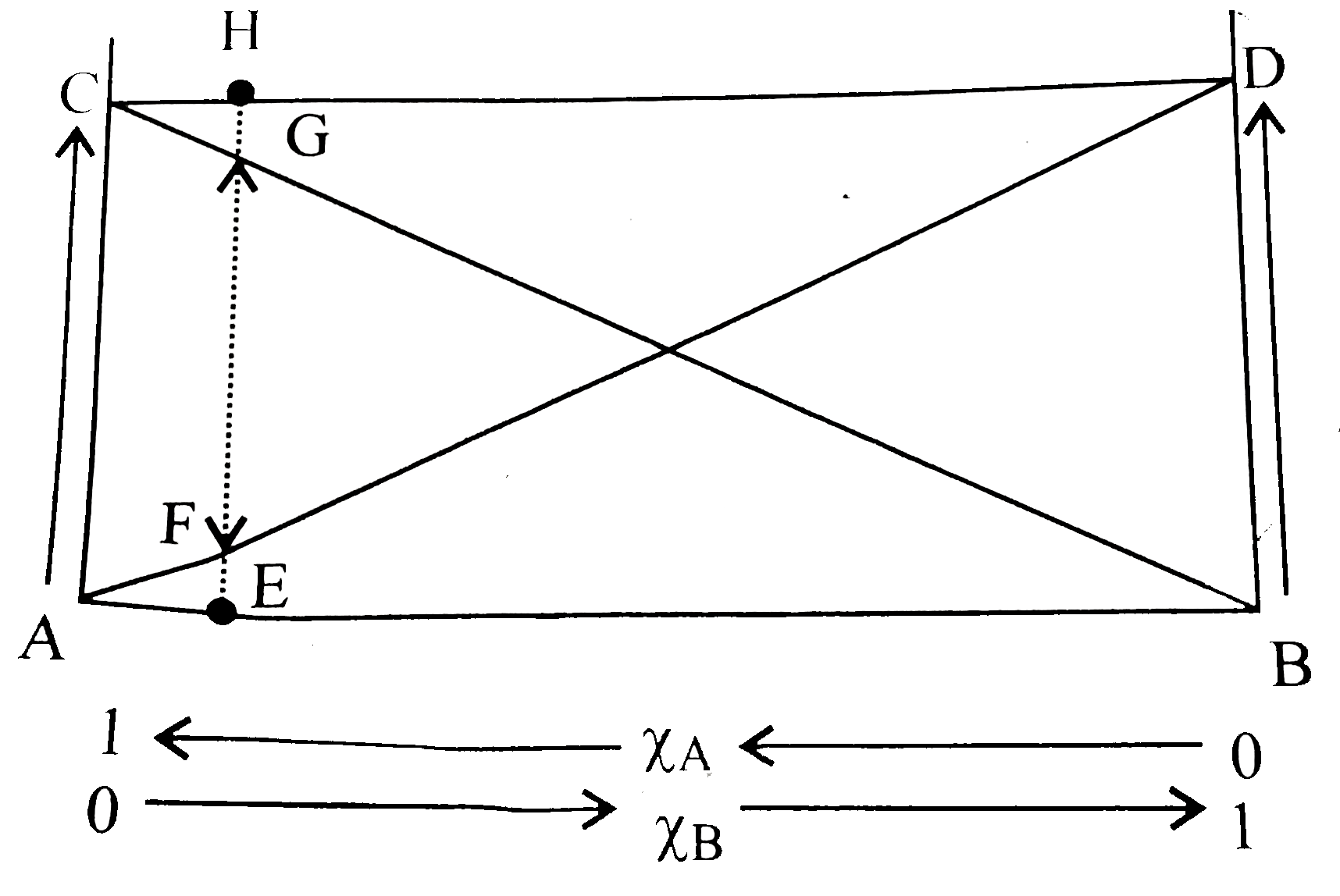
- Based on the given diagram, which of the following statements regardin...
Text Solution
|
- Based on the given diagram, which of the following statements regardin...
Text Solution
|
- Raoult's law is for solvent and Henry's law is for solute.
Text Solution
|
- Each question contains STATEMENT-I(Assertion) and STATEMENT-2(Reason)....
Text Solution
|
- Based on the given diagram, which of the following statements regardin...
Text Solution
|
- Assiertion : An ideal solution obeys Raoult's Law. Reason : In and i...
Text Solution
|
- Assertion : An ideal solution obeys Raoults Law Reason: In an ideal so...
Text Solution
|
- Assertion : An ideal solution obeys Raoults Law Reason: In an ideal ...
Text Solution
|
- State Raoult's law for solutions containing non-volatile solutes in vo...
Text Solution
|