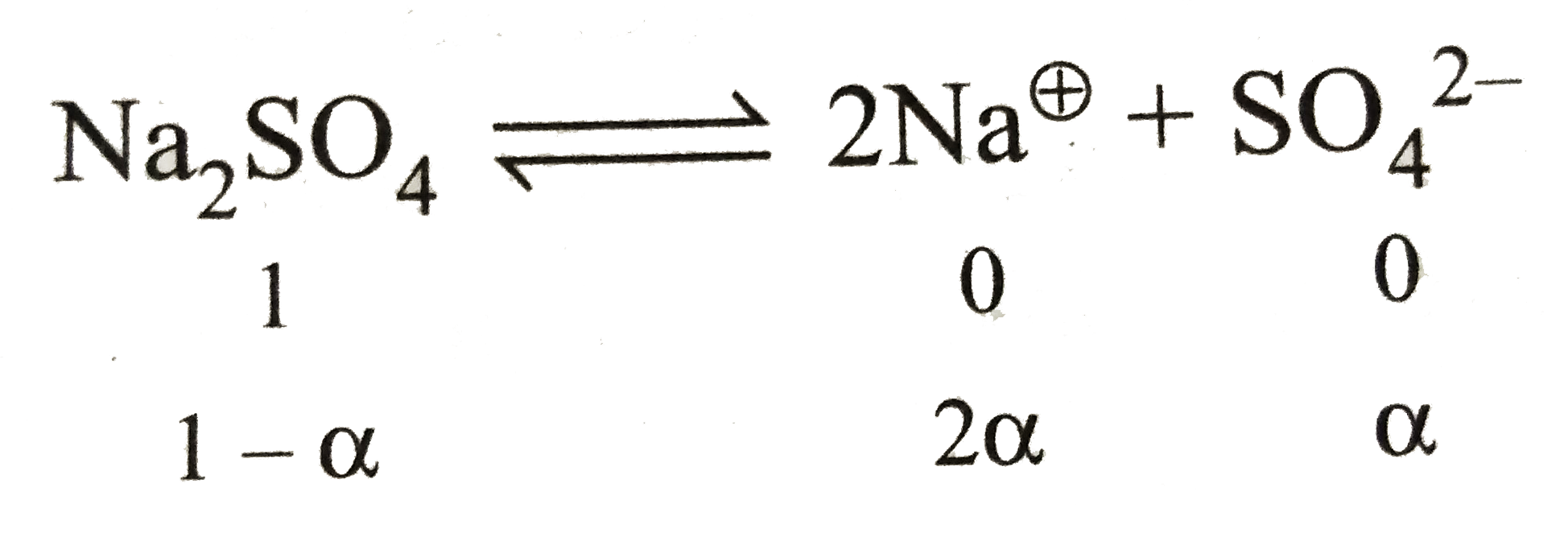A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- When 0.004 M Na(2)SO(4) is an isotonic acid with 0.01 M glucose, the d...
Text Solution
|
- A 0.004 M solution of Na(2)SO(4) is isotomic with a 0.01 M solution of...
Text Solution
|
- A 0.004M solution of Na(2)SO(4) is isotonic with a 0.010 M solution of...
Text Solution
|
- If 0.04 M Na(2)SO(4) solutions at 300K is found to be isotonic with 0....
Text Solution
|
- A 0.004 M solution of Na(2)SO(4) is isotonic with a 0.010 M solution o...
Text Solution
|
- 0.004 M Na(2)SO(4) is isotonic with 0.01 M glucose. Degree of dissocia...
Text Solution
|
- A 0.004M solution of Na(2)SO(4) is isotonic with a 0.010M solution of ...
Text Solution
|
- A 0.004 M solution of Na(2)SO(4) is isotomic with a 0.01 M solution of...
Text Solution
|
- A 0.004 M solution of Na(2)SO(4) is isotonic with 0.010 M solution of ...
Text Solution
|