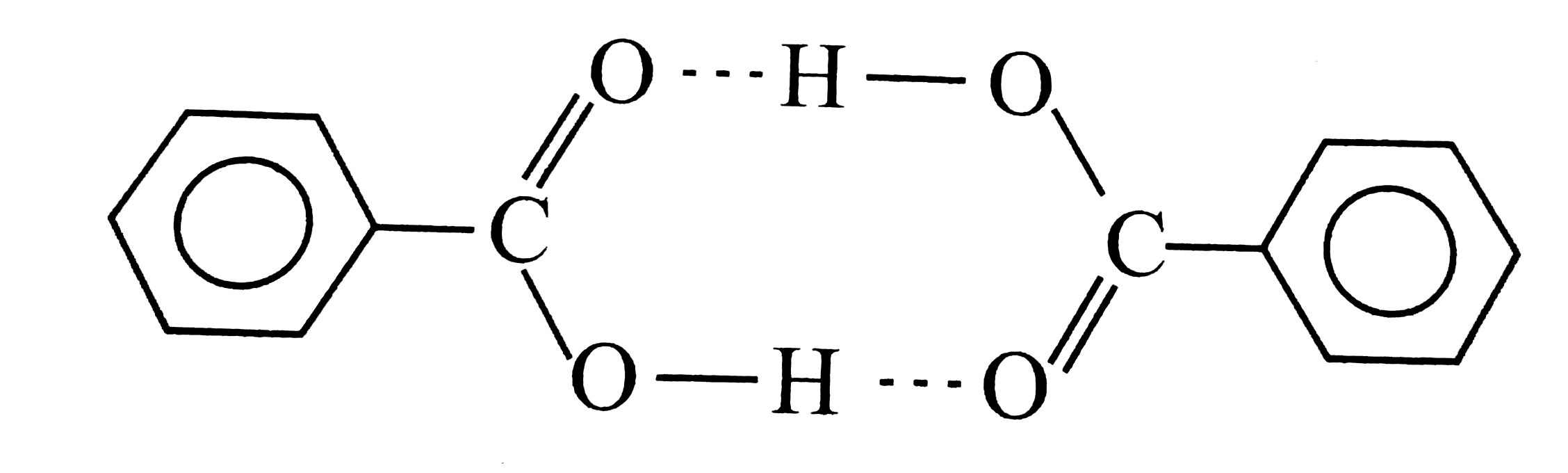A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The molecular weight of benzoic acid in benzene as determined by depre...
Text Solution
|
- From a measurement of the freezing point depression of benzene, the mo...
Text Solution
|
- The molecular weight of benzoic acid in benzene as determined by depre...
Text Solution
|
- (A) The molecular weight of acetic acid determined by depression in fr...
Text Solution
|
- हिमांक में अवनमन विधि के द्वारा निर्धारित बैन्जोइक अम्ल का बैन्जीन में...
Text Solution
|
- Assertion. The molecular weight of acetic acid determined by depressio...
Text Solution
|
- The molecular weight of benzoic acid in benzene as determined by depre...
Text Solution
|
- हिमांक में अवनमन विधि (depression in freezing point method) द्वारा ज्ञ...
Text Solution
|
- बेन्जोइक अम्ल का बेन्जीन में हिमांक अवनमन (depression in freezing poin...
Text Solution
|