Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
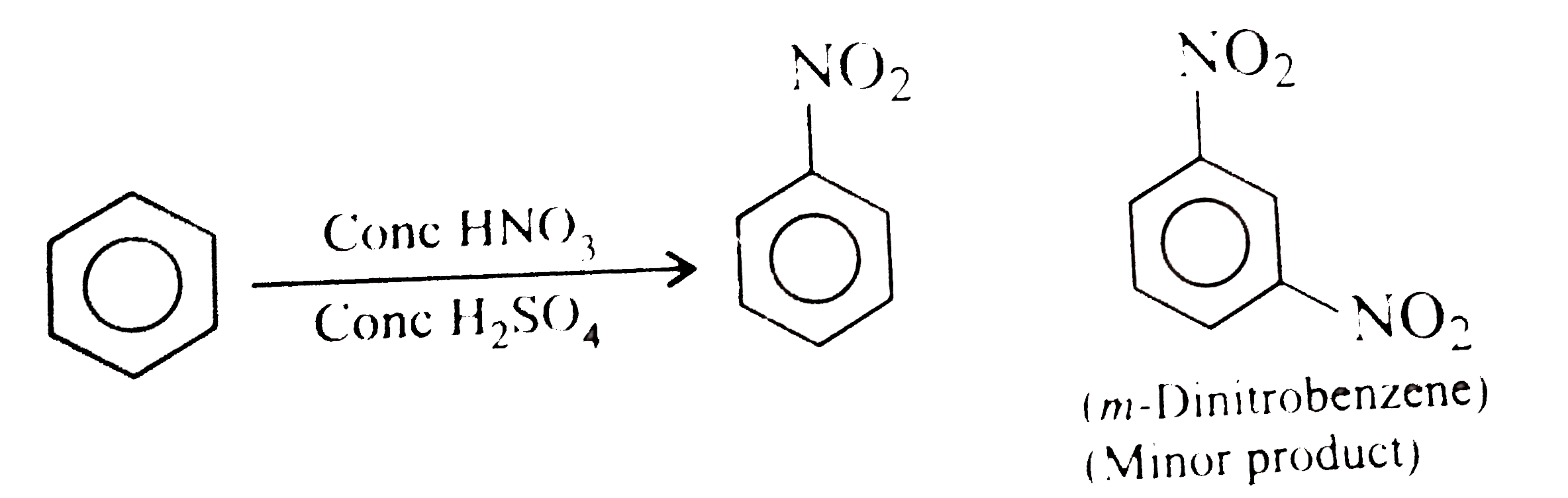
- Nitrobenzene is formed as the major product along with a minor product...
Text Solution
|
- Nirtobenzene is formed as the major product along with a minor product...
Text Solution
|
- Nitrobezene is formed as the major product along with a minor product ...
Text Solution
|
- Give the major and minor products of the dehydration of the given comp...
Text Solution
|
- Give the major and minor products.
Text Solution
|
- The product of the Reimer-Tiemann reaction is amixture of o- hydroxybe...
Text Solution
|
- The minor product obtained in the acid-catalyzed dimerization of methy...
Text Solution
|
- Identify the major and minor products in the following reactions
Text Solution
|
- Identify the major and minor products in the following reactions
Text Solution
|