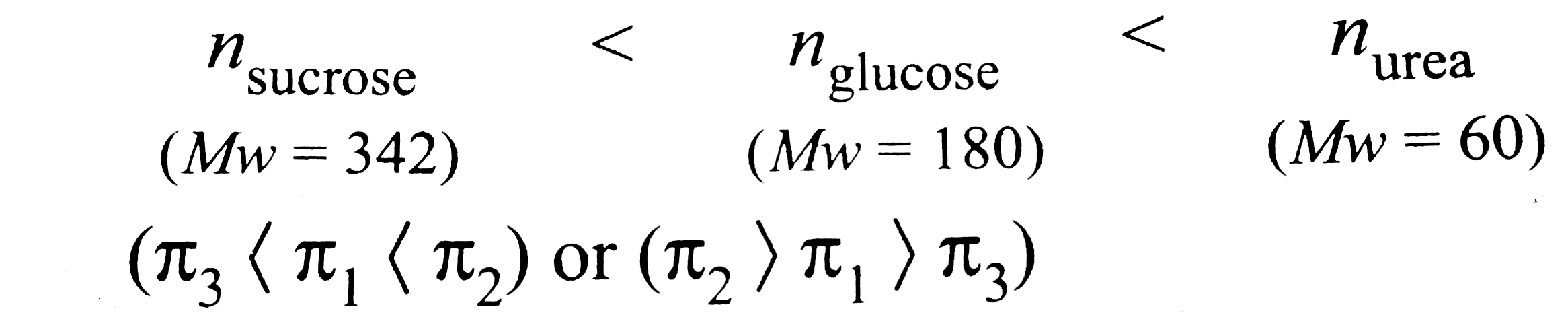A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 10.0 g of glucose (pi(1)), 10.0 g of (urea(pi(2)), and 10.0 g of sucro...
Text Solution
|
- 10.0 g of glucose (pi(1)), 10.0 g of (urea(pi(2)), and 10.0 g of sucro...
Text Solution
|
- The relationship between osmotic pressure at 273 K when 10 g glucose (...
Text Solution
|
- The relationship between osmotic pressure (pi(1),pi(2) "and" pi(3)) at...
Text Solution
|
- Relationship between osmotic pressure at 273 K when 1% glucose (pi(1))...
Text Solution
|
- The relationsip between the values of osmotic pressure of solutions ob...
Text Solution
|
- What is the relation in the osmotic pressure at 273 K when 10 g of glu...
Text Solution
|
- A solution with osmotic pressure pi(1) is separated from another solut...
Text Solution
|
- If pi(1) is the osmotic pressure of solution containing 6 g. of acetic...
Text Solution
|