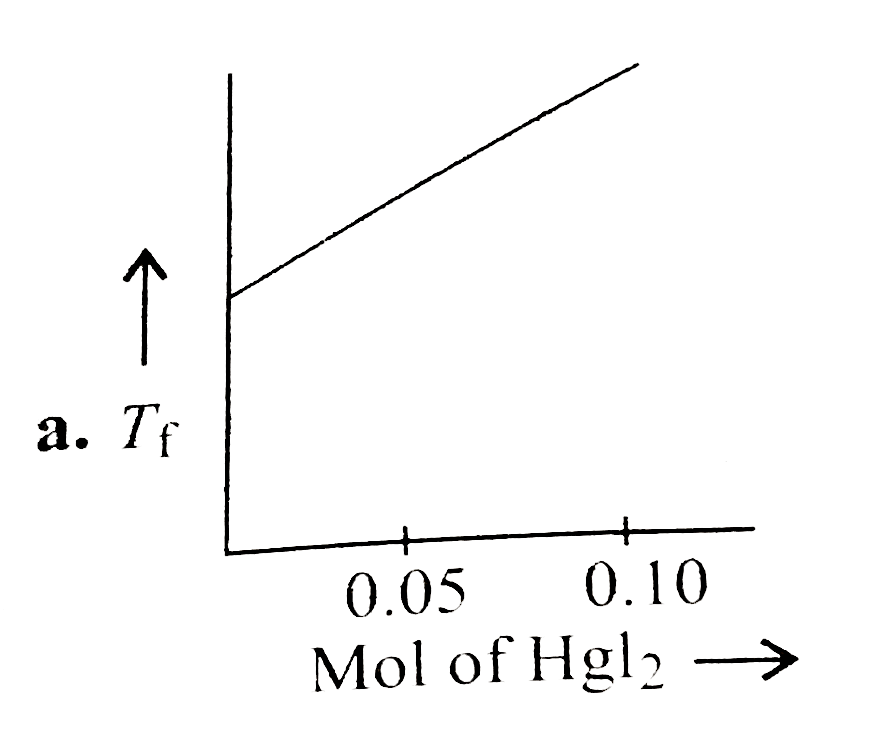
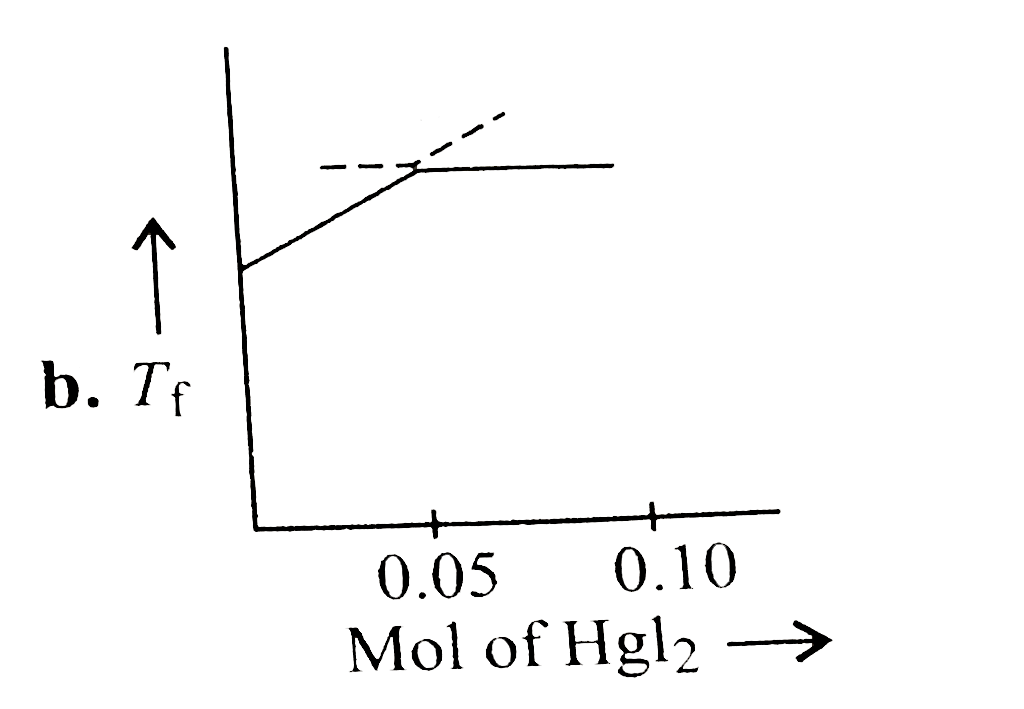
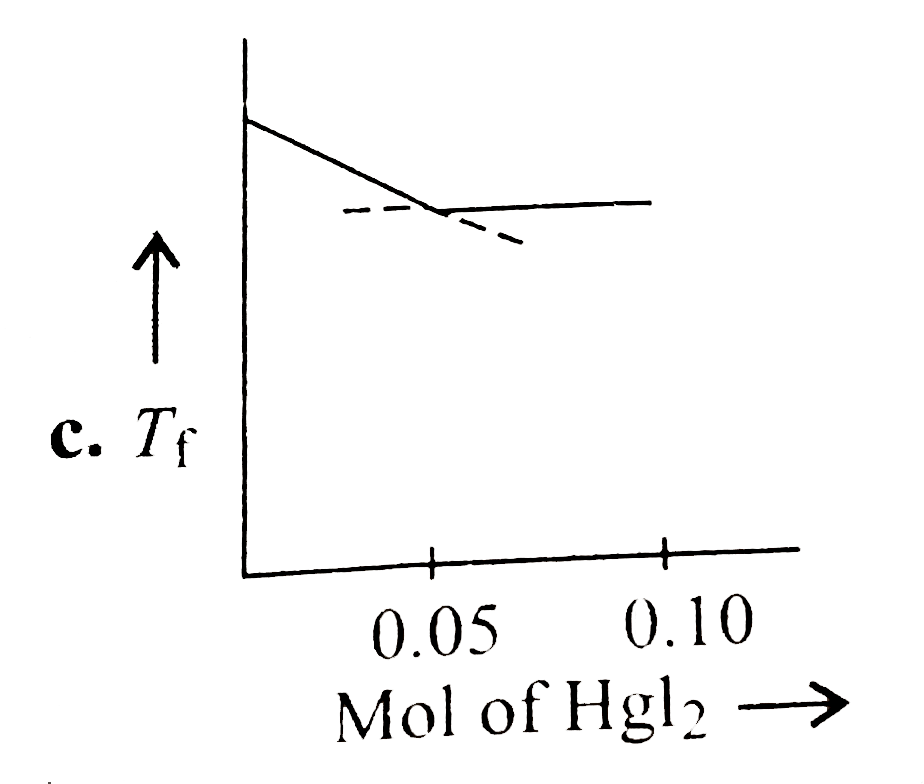
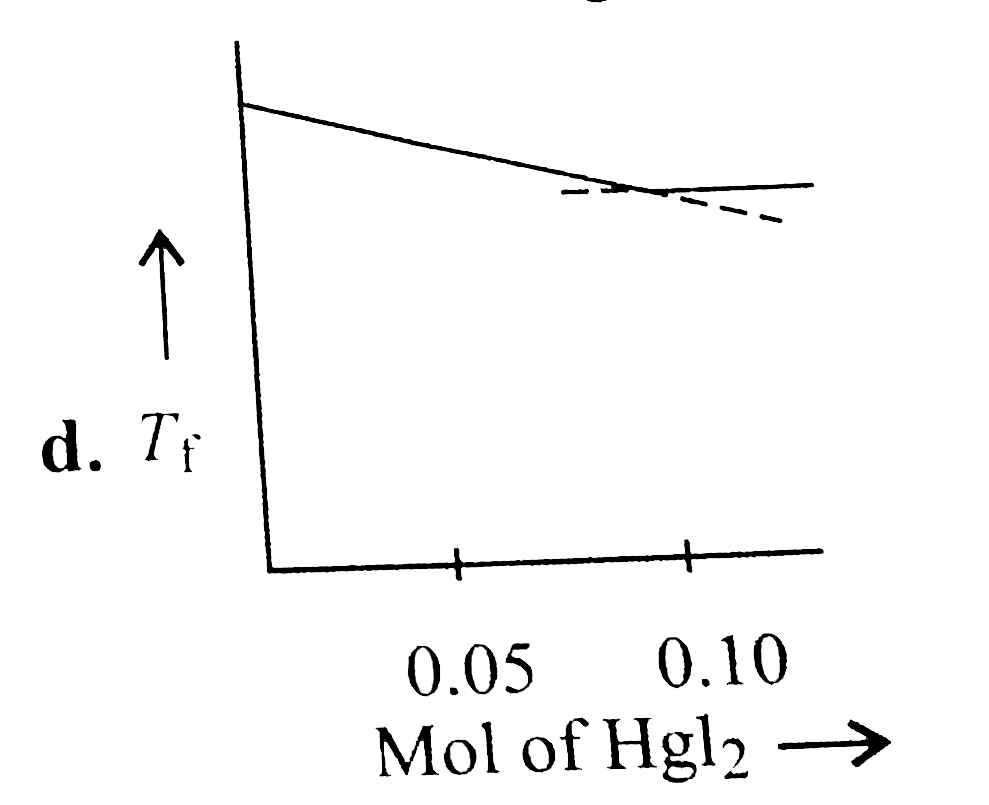
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Increasing amount of solid Hgl(2) is added to 1 L of an aqueous soluti...
Text Solution
|
- To aqueous solution of Nal , increasing amounts of solid Hgl(2) , is a...
Text Solution
|
- Statement-1 : Addition of Hgl(2) is aqueous solution of KI increases t...
Text Solution
|
- When HgI(2) is added in KI solution. The freezing point of solution:-
Text Solution
|
- STATEMENT-1 : When Hgl(2) is added to the aqueous solution of Kl, the ...
Text Solution
|
- सोडियम क्लोराइड की वह मात्रा बताएँ जो एक किलोग्राम जल में डाली जाये तो...
Text Solution
|
- The freezing point of an aqueous solution of KNC containing 0.1892 "mo...
Text Solution
|
- The amount of iodine that liberates in the reaction of 0.1 mol K(2)Cr(...
Text Solution
|
- গ্লুকোজের 0.1 M জলীয় দ্রবণের হিমাঙ্ক -0.18C। এই দ্রবণে সম-আয়তনের 0.02M...
Text Solution
|