Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- If excess of Zn is added to 1.0M solution of CuSO(4) , find the concen...
Text Solution
|
- Construct a cell using given electrodes at 298K and also calculate its...
Text Solution
|
- Calculate the maximum work that can be obtained from the decimolar Dan...
Text Solution
|
- The standard oxidation potential of Zn referred to SHE is 0.76V and th...
Text Solution
|
- If excess of Zn is added to 1.0M solution of CuSO4 , find the concentr...
Text Solution
|
- For the given cell arrangement identify incorrect statement given E^@(...
Text Solution
|
- For the given cell arrangement identify incorrect statement given E^...
Text Solution
|
- What is nernst equation. Calculate the EMF of the galvanic cell constr...
Text Solution
|
- The emf of galvanic cell of the reaction Zn+Cu^(2+)rarr Zn^(2+) + Cu i...
Text Solution
|
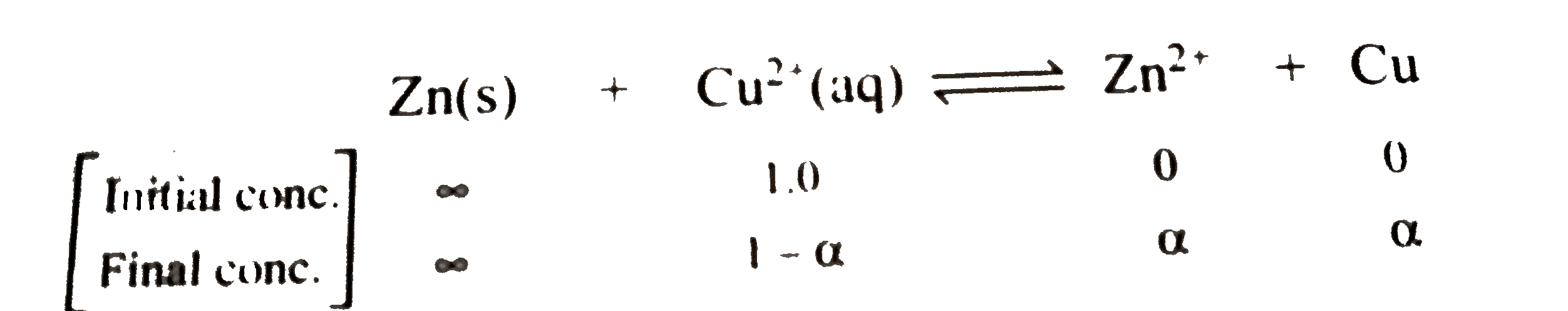 `K_(eq)=([Zn^(2+)])/([Cu^(2+)])=(alpha)/(1-alpha)~~(1)/(1-alpha)` `[:'a~~1]`
`K_(eq)=([Zn^(2+)])/([Cu^(2+)])=(alpha)/(1-alpha)~~(1)/(1-alpha)` `[:'a~~1]`