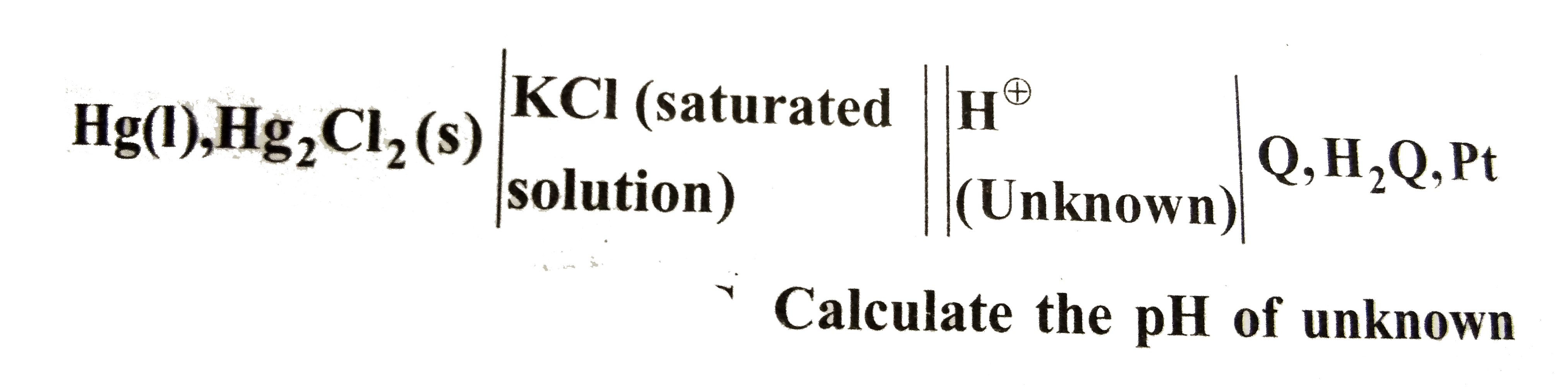Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The quinhydrone electrode (Q,H^(o+)|H^(2)Q) is used in conjunction wit...
Text Solution
|
- A saturated calomel electrode is coupled through a salt bridge with a ...
Text Solution
|
- Find the EMF of the cell at 25^(@)C . E^(c-).(red("quinhydrone electro...
Text Solution
|
- The EMF of the cell : Pt|Ce^(4+)(90%),Ce^(3+)(10%)| Normal calomel ele...
Text Solution
|
- The cell Pt, H(2) (1atm) H^(+) (pH =x)| Normal calomel Electrode has a...
Text Solution
|
- The cell, Pt|H(2)" (1 atm)"||H^(+) (pH=x)| normal calomel electrode, h...
Text Solution
|
- The cell Pt, H(2)(1atm)|H^(+)(pH=x)|| normal calomel electrode has an ...
Text Solution
|
- The calomel electrode is represented by
Text Solution
|
- The calomel electrode can be represented as .
Text Solution
|