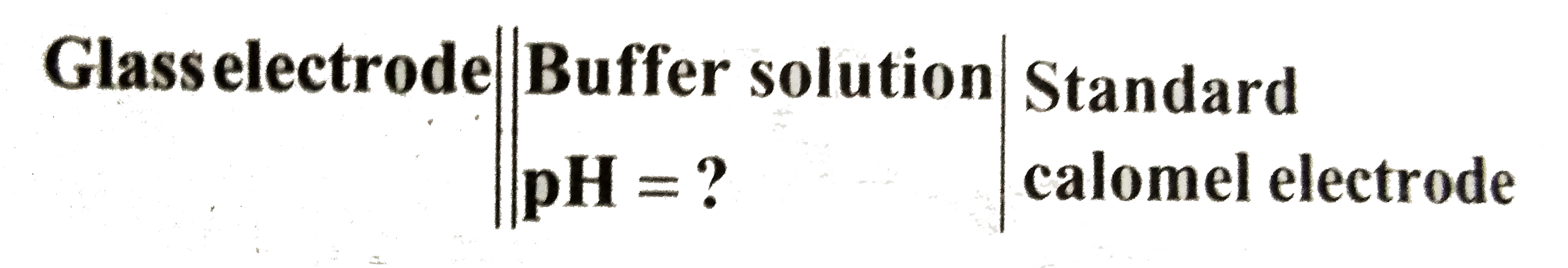Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- If the EMF of the above cell is 0.03V,E(SC E)=0.24V,E^(c-).(glass)=0.5...
Text Solution
|
- The quinhydrone electrode (Q,H^(o+)|H^(2)Q) is used in conjunction wit...
Text Solution
|
- E^(c-).(cell) of the reaction above in Question is
Text Solution
|
- In which of the following cells, EMF is greater than E^(c-).(cell)?
Text Solution
|
- Deduce from the following E^(c-) values of half cells, what combinatio...
Text Solution
|
- The correct value of emf of cell is given by (i) E("cell") = E(OP) and...
Text Solution
|
- The emf of the cell : H(2)(g)|"Buffer||Normal" canlomel elctrolde is 0...
Text Solution
|
- The pH of an acidic buffer solution & a basic buffer solution at 25^(@...
Text Solution
|
- The temperature dependence of the emf of a standard electrochemical ce...
Text Solution
|