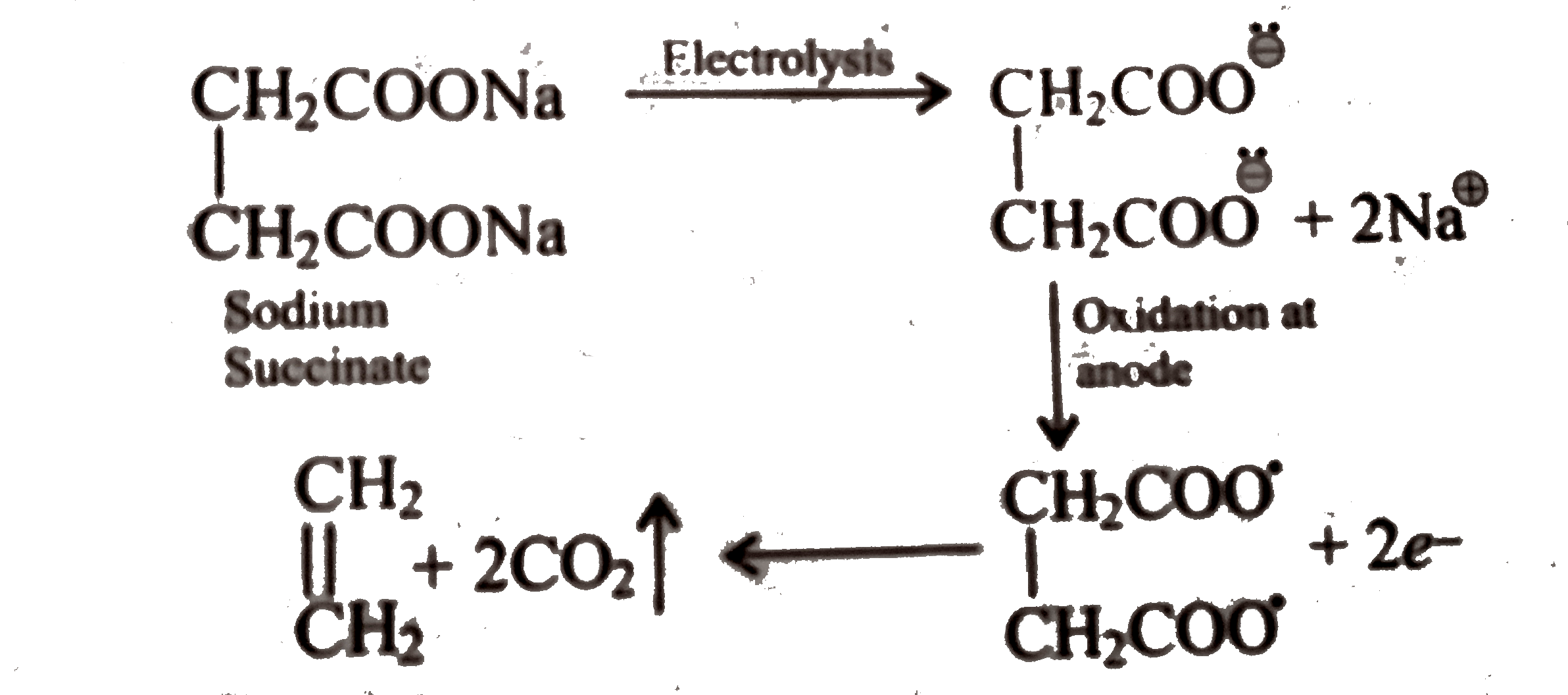
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Find the volume of gases evolved by passing 0.1 A of current for 965s...
Text Solution
|
- A current of 5.0 A is passed through a 100L aqueous solution of sodium...
Text Solution
|
- The volume of gases evolved at STP by passing 0.1A of current for 965g...
Text Solution
|
- The volume of gases evolved at STP by passing 0.2A of current for 965 ...
Text Solution
|
- i. The volume of gases evolved at STP by passing 0.1 A of current for ...
Text Solution
|
- Find the volume of gases evolved by passing 0.9655 . A current for 1 h...
Text Solution
|
- When an electric current is passed through an aqueous solution of sodi...
Text Solution
|
- When 965 amp current is passed through aqueous solution of salt X usin...
Text Solution
|
- Aqueous solution of sodium succinate on electrolysis gives mainly
Text Solution
|