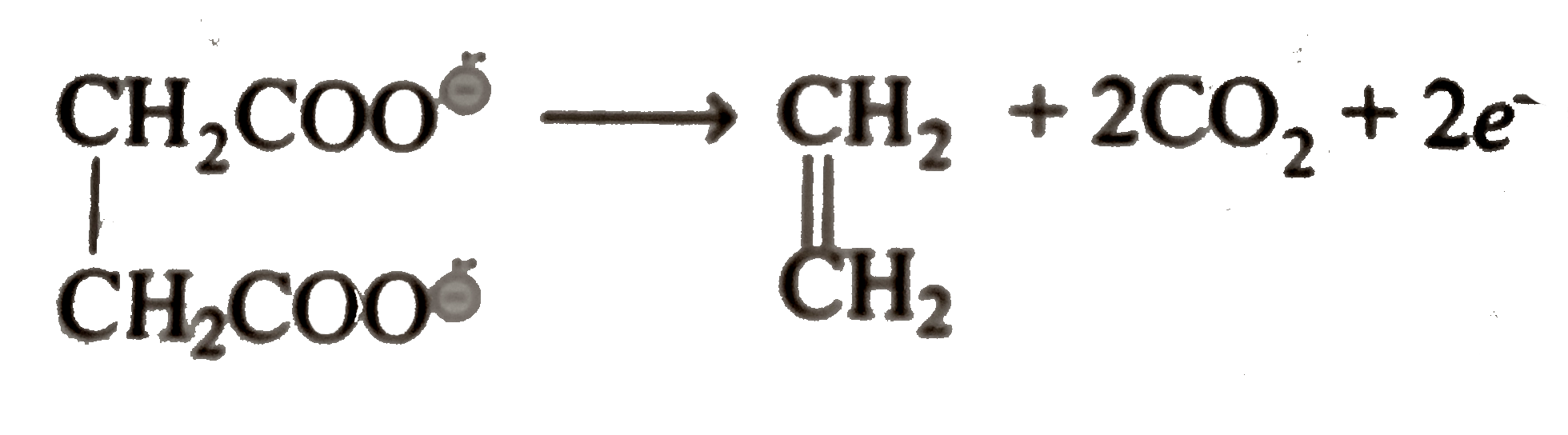Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A current of 5.0 A is passed through a 100L aqueous solution of sodium...
Text Solution
|
- In the electrolysis of 7.2L aqueous solution of CuSO(4) , a current of...
Text Solution
|
- Find the volume of gases evolved by passing 0.1 A of current for 965s...
Text Solution
|
- A current strength of 1.0 A is passed for 96.5s through 200mL of a sol...
Text Solution
|
- A current of 1.0A is passed for 96.5s trhough a 200mL solution of 0.05...
Text Solution
|
- Find the volume of gases evolved by passing 0.9655 . A current for 1 h...
Text Solution
|
- Calculate the pH of 0.5 of 1.0 M NaCl solution after electrolysis when...
Text Solution
|
- At 25^(@)C the pH value of a solution is 6. The solution is
Text Solution
|
- pH of a solution produced when an aqueous solution of pH 6 is mixed wi...
Text Solution
|