Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The standard potential of a cell using the reaction is 1.12. The heat...
Text Solution
|
- Rate constant of a reaction changes by 2% by 0.1^(@)C rise in temperat...
Text Solution
|
- The standard free energy change for a reaction is -213.3 KJ mol^(-1) "...
Text Solution
|
- The standard free energy change for a reaction is -212.3 kJ mol^(-1). ...
Text Solution
|
- At 25^(@)C the standard heat of formation of liquid H(2)O is -286.0 kJ...
Text Solution
|
- The standard heat of formation of H2O((l)) from its elements is –285.8...
Text Solution
|
- Standard enthalpy change for combustion of methane is –890 kJ "mol"^(-...
Text Solution
|
- The standard heat of formation of H2O((l)) from its elements is –285.8...
Text Solution
|
- Standard enthalpy change for combustion of methane is –890 kJ "mol"^(-...
Text Solution
|
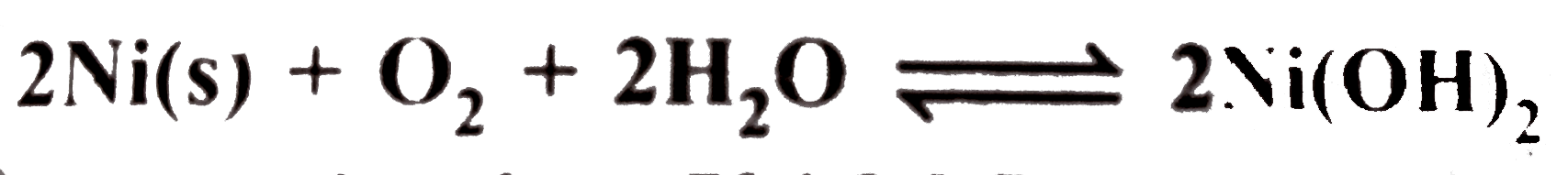 is `1.12`. The heat of the reaction is `-504.2kJ mol ^(-1)` at `25^(@)C`. Calculate the entropy change.
is `1.12`. The heat of the reaction is `-504.2kJ mol ^(-1)` at `25^(@)C`. Calculate the entropy change.