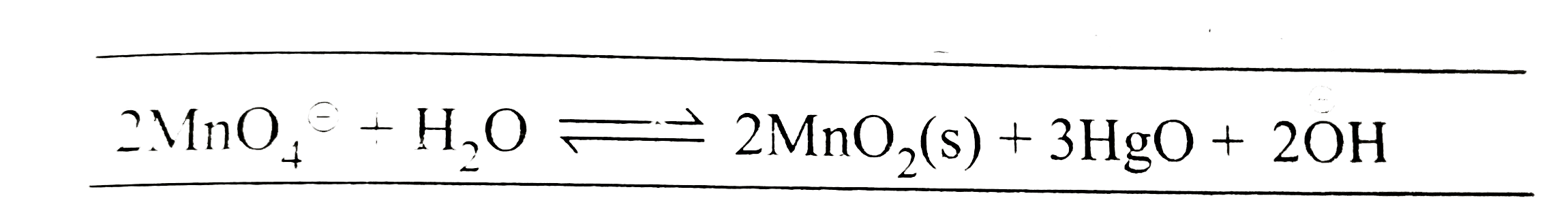Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The standard potential of a cell using the reaction +3HgO(s)+2(overse...
Text Solution
|
- The standard reduction potential at 25^(@)C for the reaction, volt. Th...
Text Solution
|
- The standard reduction potential at 25^(@)C of the reaction 2H(2)O+2e^...
Text Solution
|
- For a cell reaction involving a two-electron change, the standard e.m...
Text Solution
|
- E^(@) for the cell Zn(s)|Zn^(2+)(aq)|Cu^(2+)(aq)|Cu(s) is 1.1V at 25^(...
Text Solution
|
- एक सेल अभिक्रिया जिसमे दो इलेक्ट्रॉनों का परिवर्तन होता है में सेल का ...
Text Solution
|
- The standard emf for the cell reaction, 2Cu^(+)(aq)to2Cu(s)+Cu^(2+)(aq...
Text Solution
|
- At 25^(@)C equilibrium constant for the reaction Ni((s))+2Ag((aq))^(+)...
Text Solution
|
- The standard reduction potential at 25^(@)C of the reaction 2H(2)O+2...
Text Solution
|
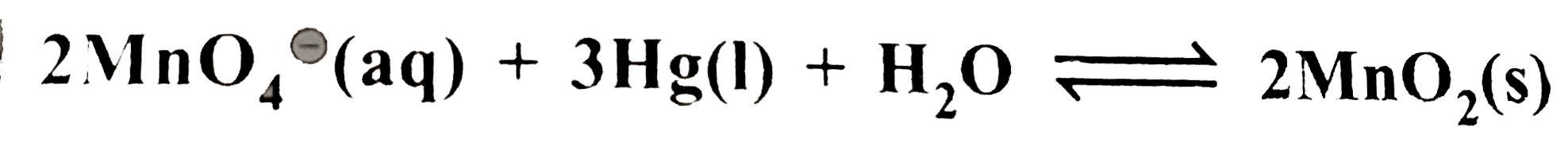 `+3HgO(s)+2(overset(c-)(O)H) (aq)` is `0.489V` at `25^(@)C`. What is the equilibrium constant of the reaction ?
`+3HgO(s)+2(overset(c-)(O)H) (aq)` is `0.489V` at `25^(@)C`. What is the equilibrium constant of the reaction ?