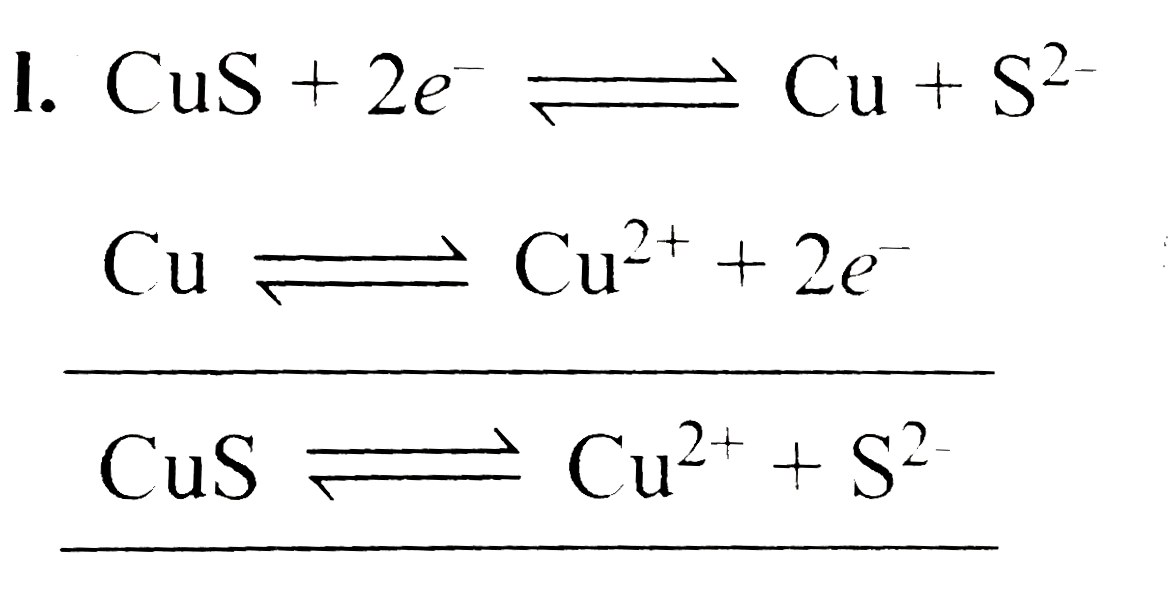Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Estimate the E^(c-) reduction for Cu|CuS electrode. Given : K(sp) o...
Text Solution
|
- Estimate the E^(c-) reduction for Cu|CuS electrode. Given : K(sp) o...
Text Solution
|
- What is E^(c).(red) for the reaction : Cu^(2+)+2e^(-) rarr Cu in the h...
Text Solution
|
- Given: (i) Cu^(2+)+2e^(-) rarr Cu, E^(@) = 0.337 V (ii) Cu^(2+)+e^(-) ...
Text Solution
|
- Given: (i) Cu^(2+)+2e^(-) rarr Cu, E^(@) = 0.337 V (ii) Cu^(2+)+e^...
Text Solution
|
- Calculate the standard reduction potential of the following half cell ...
Text Solution
|
- At 25^(@)C, the reduction potential of Cu^(2+)|Cu half - cell is 0.28V...
Text Solution
|
- If E(Cu^(2+)|Cu^(+))^(@)=+0.15V and E(Cu^(2+)|Cu)^(@) and E(Cu^(2+)|Cu...
Text Solution
|
- Given that K(sp) of CuS=10^(-35) and ECu//Cu^(2+)= (-0.34V). The stan...
Text Solution
|