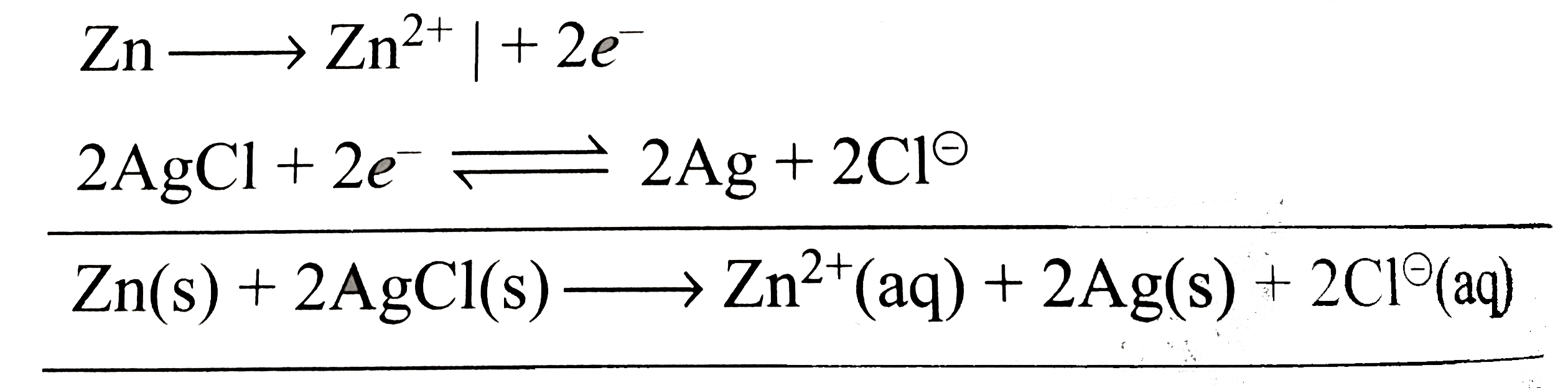Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the cell Zn|ZnCl(2)(m)|AgCl,E is 1.24V at 25^(@)C and 1.260V at 35...
Text Solution
|
- The EMF of Westron standard cell is 1.0153 at 20^(@)C and 1.01807 at 2...
Text Solution
|
- The standard potential of the following cell is 0.23V at 15^(@)C and 0...
Text Solution
|
- The standard emf for the cell cell reaction Zn + Cu^(2+) rarr Zn^(2+)...
Text Solution
|
- The measured e.m.f. at 25^(@)C for the cell reaction , Zn(S)+Cu^(2+)...
Text Solution
|
- The standard potential of the following cell is 0.23 V at 15^(@)C and ...
Text Solution
|
- Calculate the emf of the cell having the cell reaction 2Ag^(+)+Zn iff ...
Text Solution
|
- Calculate the E.M.F. of the zinc - silver cell at 25^(@)C when [Zn^(2+...
Text Solution
|
- Calculate the E.M.F. of the zinc - silver cell at 25^(@)C when [Zn^(2+...
Text Solution
|