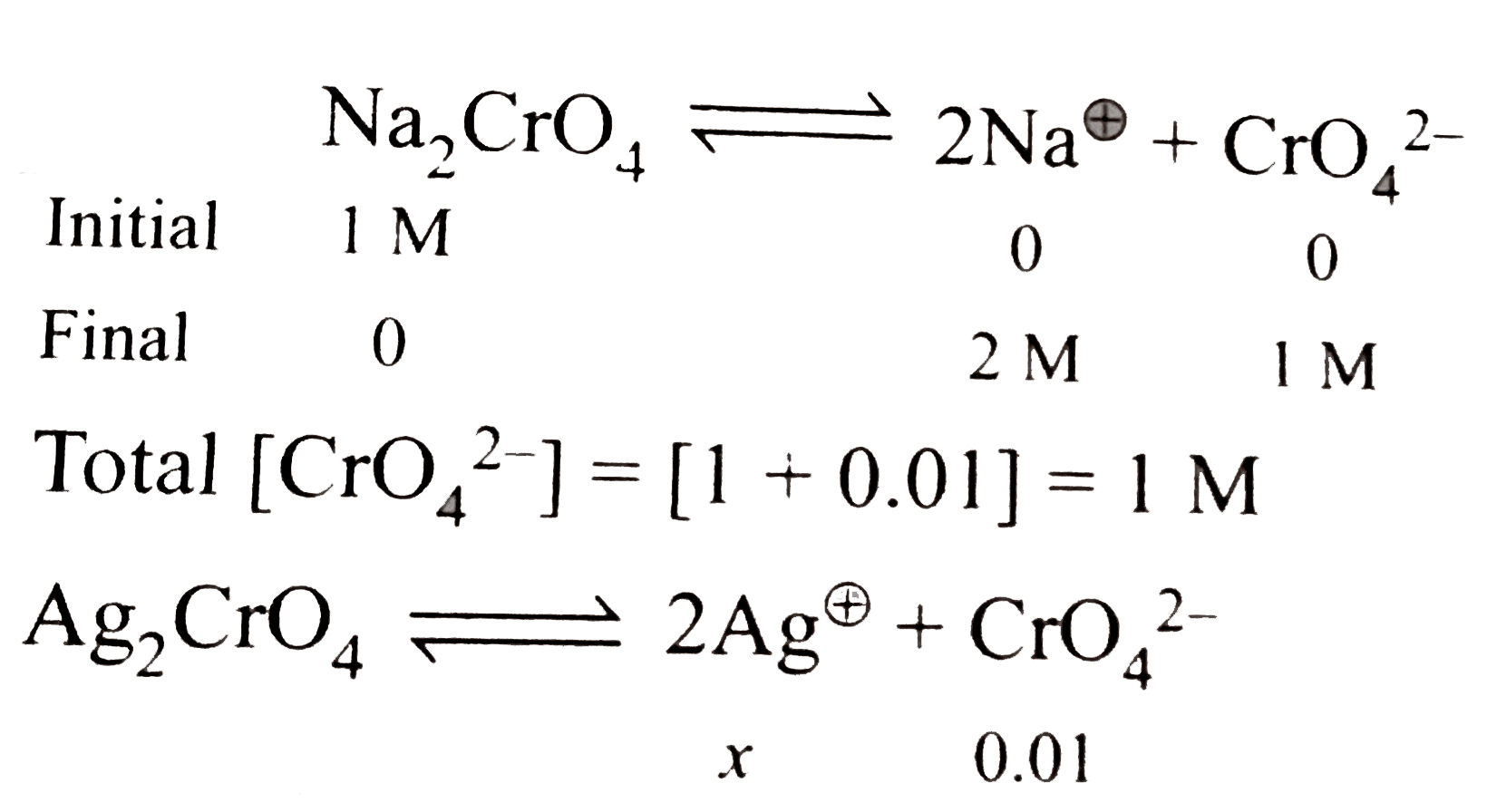Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The EMF of the cell : Ag|Ag(2)CrO(4)(s),K(2)CrO(4)(0.01 M)||AgNO(3)(...
Text Solution
|
- The EMF of the cell : Ag|Ag(2)CrO(4)(s),K(2)CrO(4)(0.1 M)||AgNO(3)(0...
Text Solution
|
- Find the solubility product of a saturated solution of Ag(2)CrO(4) in ...
Text Solution
|
- The K(sp) of Ag(2)CrO(4) is 1.1xx10^(-12) at 298K . The solubility (in...
Text Solution
|
- Solubility of Ag(2)CrO(4) ( K(sp)=4xx10^(-13) ) in 0.1 M K(2)CrO(4) so...
Text Solution
|
- The K(sp) of Ag(2)CrO(4) is 1.1xx10^(-12) at 298K. The solubility (in...
Text Solution
|
- The K(sp) of Ag(2) CrO(4) is 1.1xx10^(-12) at 298 K. The solubility (i...
Text Solution
|
- Find the solution product of Ag(2)CrO(4) in water at 298 K if the e.m....
Text Solution
|
- 298 K पर Ag(2)CrO(4) के K(sp) का मान 1.1 xx 10^(-12) है| 0.1 M AgNO(3)...
Text Solution
|