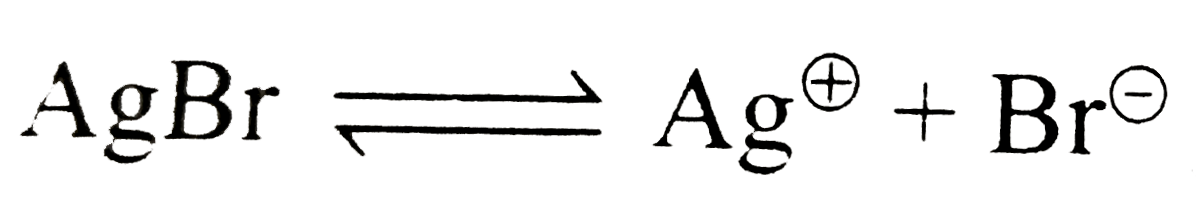
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Calculate the potential of silver electrode in a saturated solution of...
Text Solution
|
- Knowing that K(sp) for AgCl is 1.0 xx 10^(-10), calculate E for a silv...
Text Solution
|
- What would be the electrode potential of a silver electrode dipped in ...
Text Solution
|
- The standard reduction potential of the Ag^(o+)|Ag electrode at 298K i...
Text Solution
|
- Calculate the cell potential, E, for a silver-silver chloride electrod...
Text Solution
|
- In a saturated aqueous solution of AgBr, concentration of Ag^(+) ion ...
Text Solution
|
- (a) Calculate the electrode potential of silver electrode dipped in 0....
Text Solution
|
- The electrode potential of a silver electrode dipped in 0.1 M AgNO(3) ...
Text Solution
|
- A silver electrode is immersed in a 0.1 (M) AgNO(3) solution at 25^(@)...
Text Solution
|