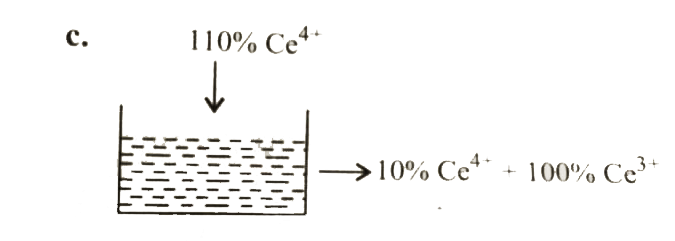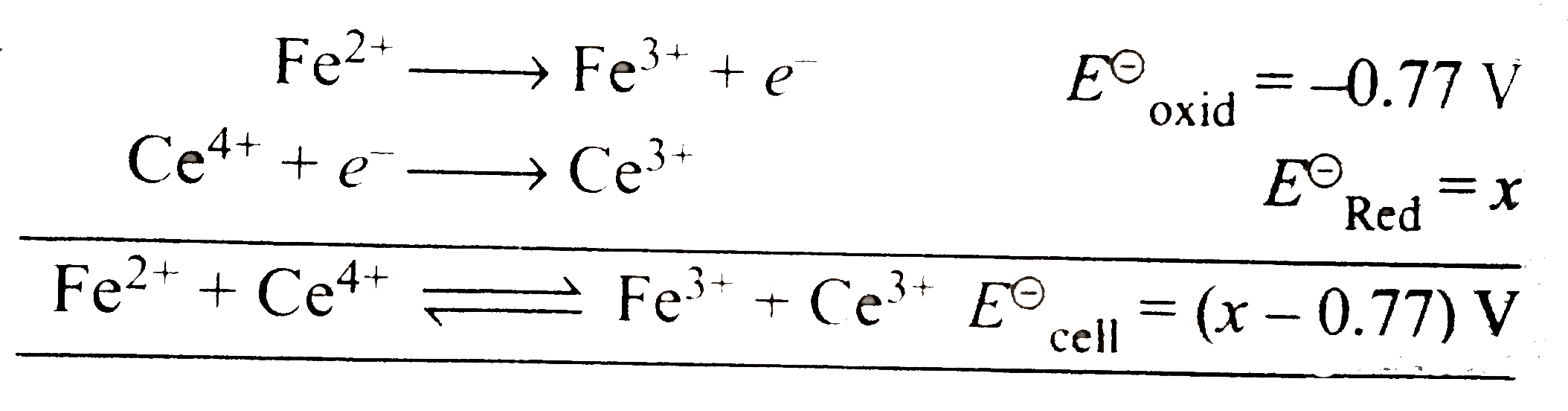Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A solution of Fe^(2+) is titrated potentiaometrically using Ce^(4+) so...
Text Solution
|
- A solution of Fe^(2+) is titrated potentiaometrically using Ce^(4+) so...
Text Solution
|
- An aqueous solution containing 0.1M Fe^(3+) and 0.01 M Fe^(2+) was tit...
Text Solution
|
- E(Fe^(3+)//Fe^(+2))^(0)=+0.77V,E(Fe^(+3)//Fe)^(0)=0.036V . What is E(F...
Text Solution
|
- A solution containing Fe^(2+) ions is titrated with KMnO(4) solution, ...
Text Solution
|
- When KMnO(4) solution is titrated with a solution containing Fe^(2+) i...
Text Solution
|
- A solution contains Fe^(2+), Fe^(3+) and T^(-) ions. This solution was...
Text Solution
|
- Standard electrode potentials are Fe^(2+)//Fe," "E^(@)=-0.44V Fe^(3+)/...
Text Solution
|
- एक विलयन में Fe^(2+),Fe^(3+) तथा I^(-) आयन उपस्थित है इस विलयन 35^(@)...
Text Solution
|