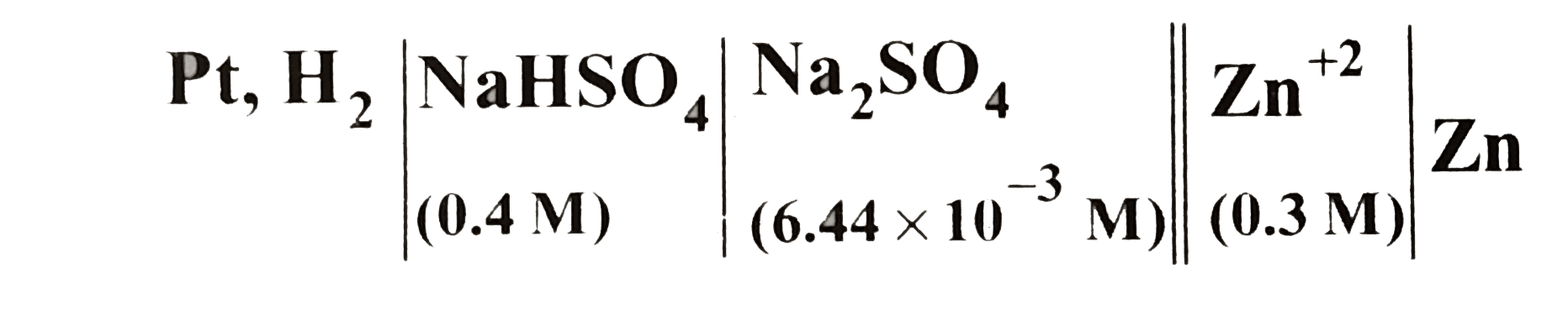Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The EMF of the following cell is found to be -0.46V: If the stan...
Text Solution
|
- The energy released in the neutralisation of H(2)SO(4) and KOH is 59.1...
Text Solution
|
- The EMF of the following cell is found to be -0.46V: If the standard e...
Text Solution
|
- Balance the following equation by oxidation number method: K(2)Cr(2)...
Text Solution
|
- Balance the following equation by oxidation number method Al+KMnO(4...
Text Solution
|
- रेडॉक्स अभिक्रिया किसे कहते है ? निम्नलिखित समीकरण को ऑक्सीकरण संख्या ...
Text Solution
|
- जल में H(2)SO(4) के लिये 1Ka(2)<<K(al) क्यों है? H(2)SO(4)hArrHSO(4)^...
Text Solution
|
- Balance the following equations : (i) KMnO(4) + H(2)SO(4) + H(2)O(2) t...
Text Solution
|
- H(2) SO(4) in aqueous medium ionises in two steps H(2) SO(4) (aq) +...
Text Solution
|