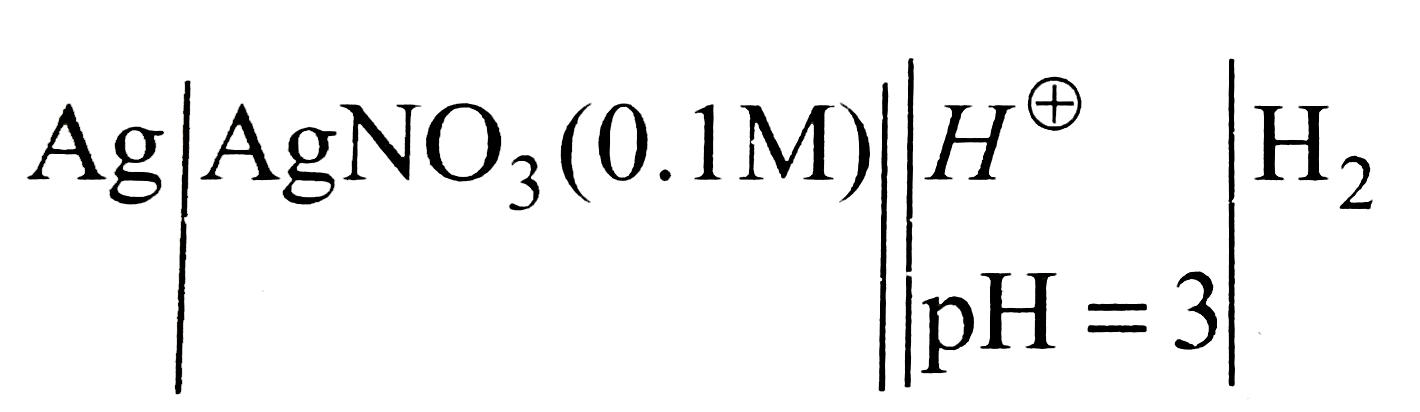Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A silver electrode dipping in AgNO(3) solution (0.1M) is combined salt...
Text Solution
|
- A silver electrode dipping in AgNO(3) solution (0.1M) is combined salt...
Text Solution
|
- If hydrogen electrodes dipped in two solutions of pH=3 and pH=6 are co...
Text Solution
|
- What is the electrode potential of a gasous hydrogen electrode dipped ...
Text Solution
|
- A hydrogen electrode is dipped in a solution at 25^(@)C. The potential...
Text Solution
|
- If hydrogen electrode dipped I 2 solution of pH=3 and pH=6 and salt br...
Text Solution
|
- (a) Calculate the electrode potential of silver electrode dipped in 0....
Text Solution
|
- सिल्वर इलेक्ट्रोड के लिये मानक अपचयन विभव +0.80 वोल्ट है । गैल्वेनिक स...
Text Solution
|
- एक हाइड्रोजन इलेक्ट्रोड को 25^@C पर pH=3 वाले विलयन में डुबाया गया ।...
Text Solution
|