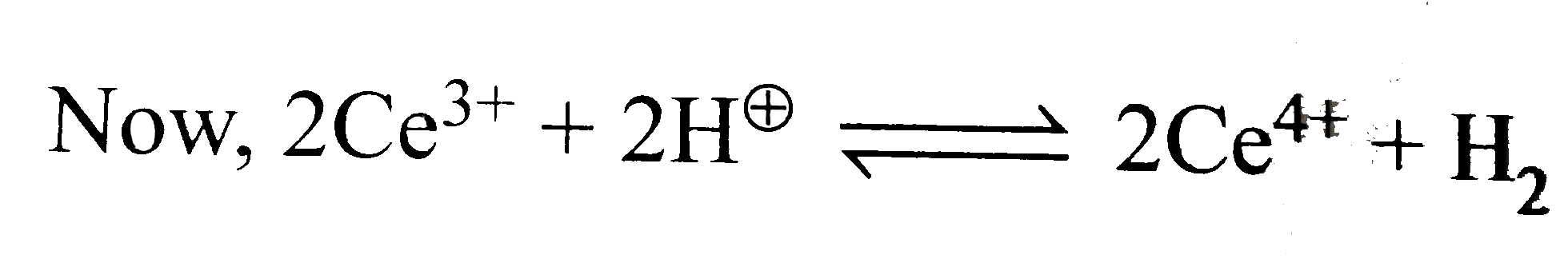
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The EMF of the cell : Pt|Ce^(4+)(90%),Ce^(3+)(10%)| Normal calomel...
Text Solution
|
- The EMF of the cell : Pt|Ce^(4+)(90%),Ce^(3+)(10%)| Normal calomel ele...
Text Solution
|
- What is the electrode potential of a gasous hydrogen electrode dipped ...
Text Solution
|
- A certain electrode has standard ( reduction potential ) of 0.384 V . ...
Text Solution
|
- For the reaction : 2Ce^(4+)+Co rarr 2Ce^(3+)+Co^(3+), E(cell)^(@) = 1....
Text Solution
|
- The e.m.f. of the obtained by combining Zn and Cu electrodes of Danie...
Text Solution
|
- The cell Pt, H(2) (1atm) H^(+) (pH =x)| Normal calomel Electrode has a...
Text Solution
|
- The cell Pt, H(2)(1atm)|H^(+)(pH=x)|| normal calomel electrode has an ...
Text Solution
|
- 2Ce^(4+)+Co to 2Ce^(3+)+Co^(2+),E("cell")^(@)=1.89V यदि E(underset(1...
Text Solution
|