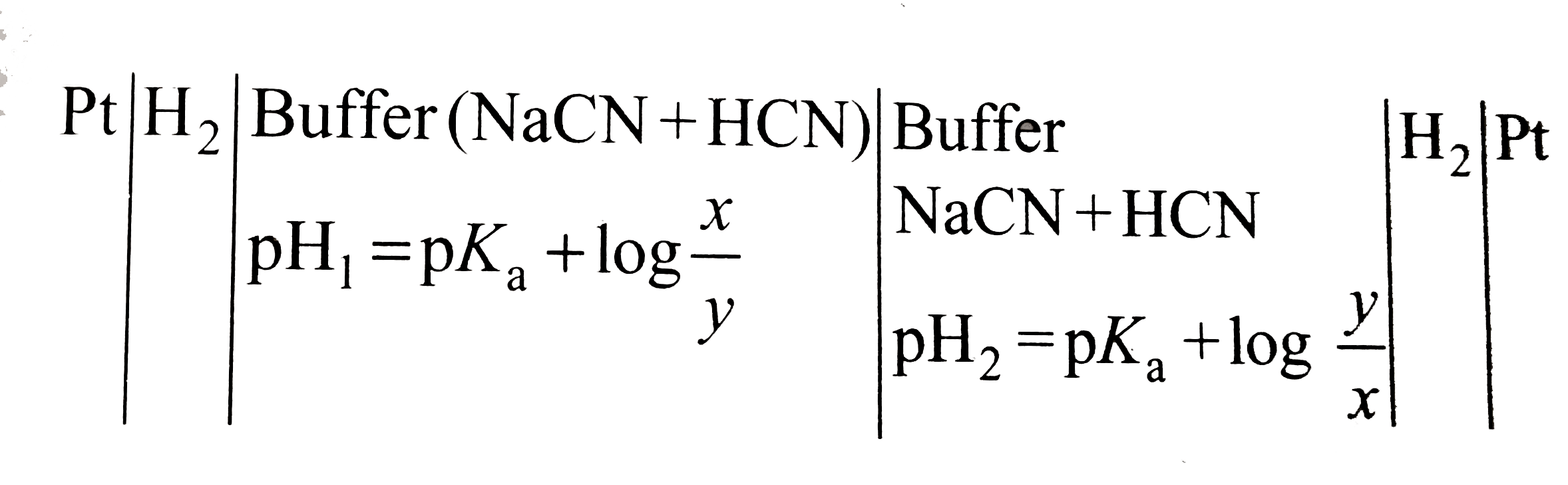Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A hydrogen electrode placed in a buffer solution of sodium cyanide and...
Text Solution
|
- A hydrogen electrode placed in a solution containing sodium acetate an...
Text Solution
|
- A hydrogen electrode placed in a solution containing sodium acetate an...
Text Solution
|
- Buffer soution'A' of a weak monoprotic acid and it's sodium salt in co...
Text Solution
|
- The reduction potential of hydrogen electrode at 25^(@) C when placed ...
Text Solution
|
- A hydrogen electrode placed in a buffer solution of CH(3)COONa and CH(...
Text Solution
|
- The reduction potential of hydrogen electrode when placed in a buffer ...
Text Solution
|
- A hydrogen electrode placed in a solution containing sodium acetate an...
Text Solution
|
- A hydrogen electrode placed in a buffec solution of CH3COONA and aceti...
Text Solution
|