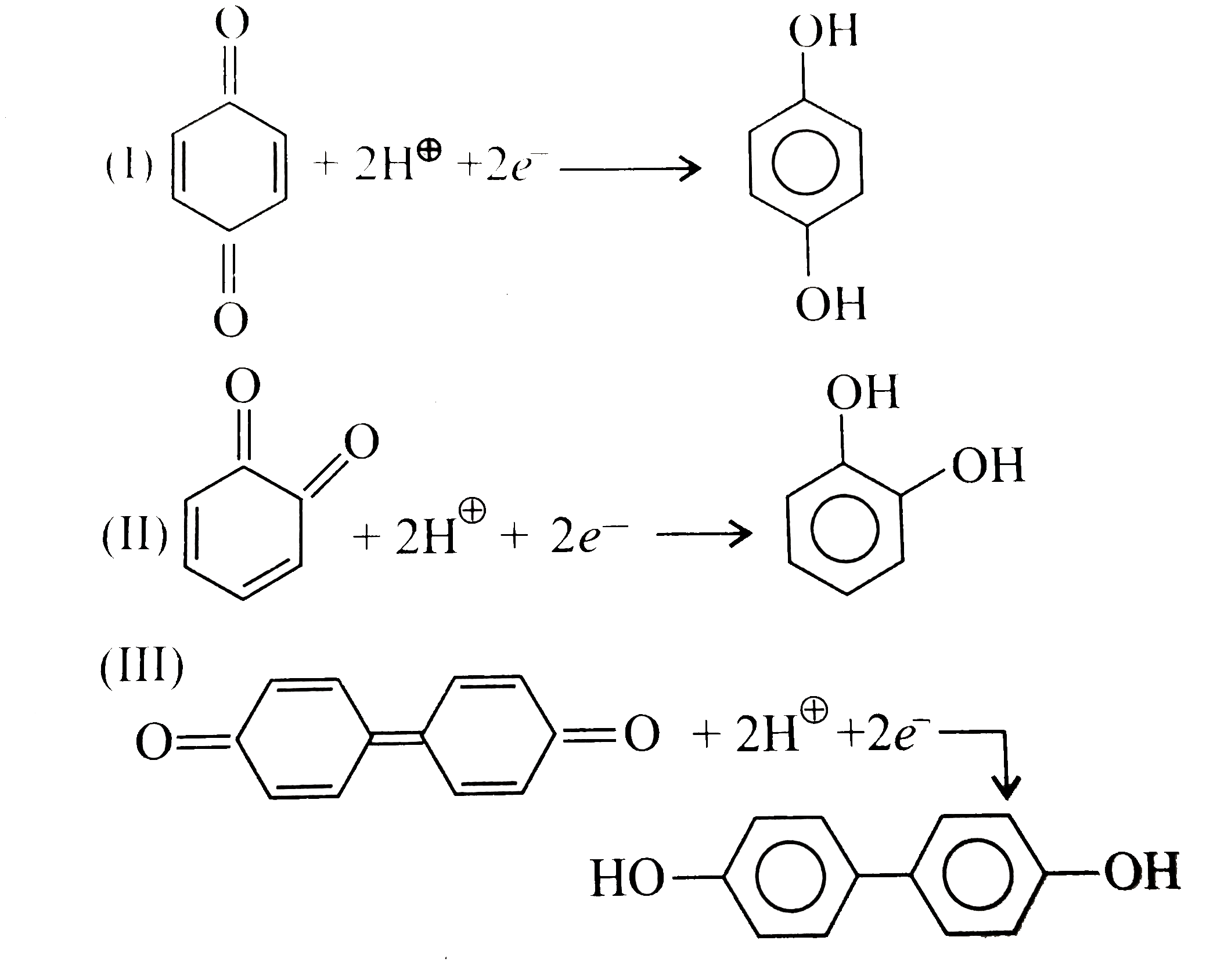
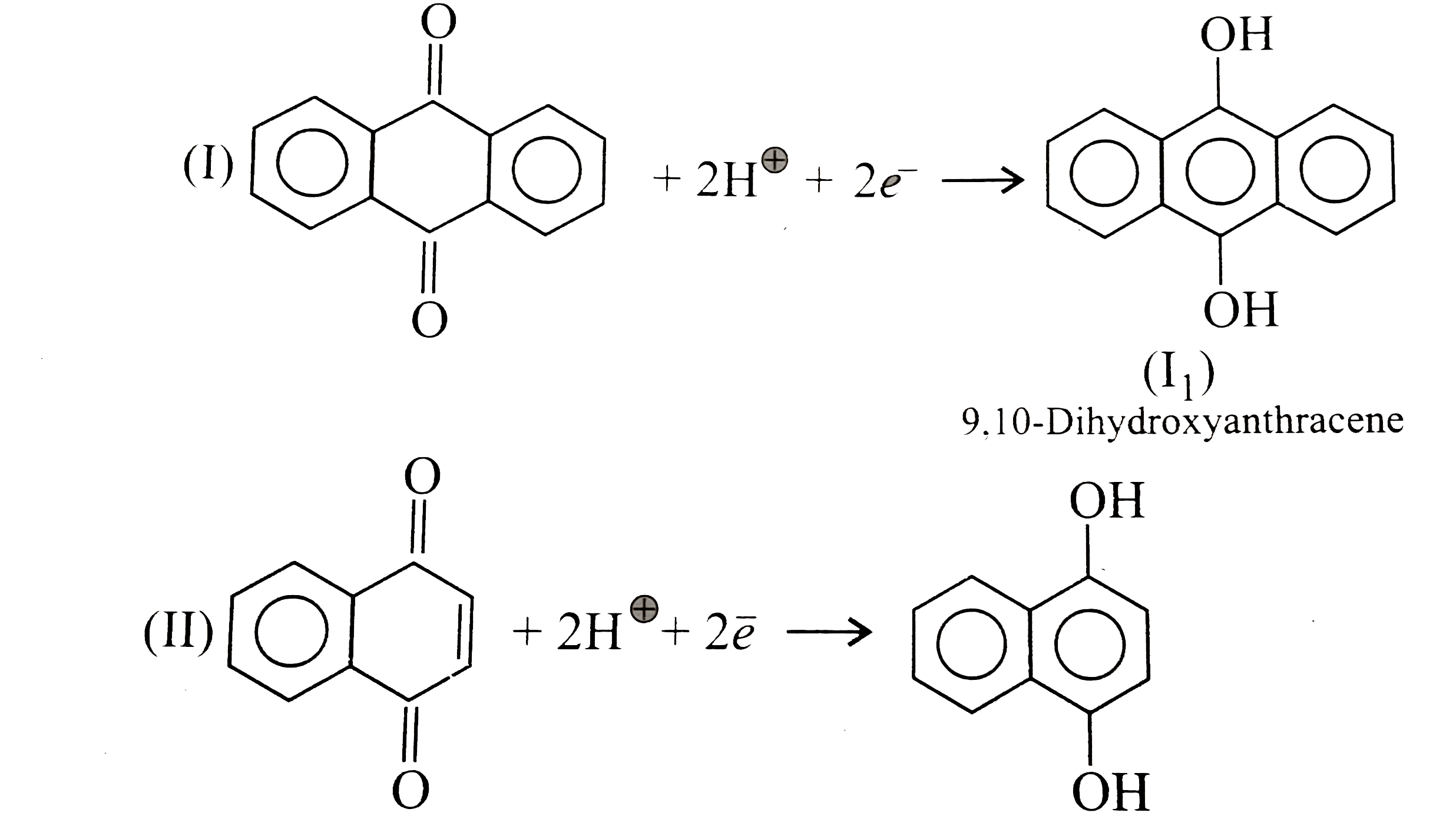
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Quinones are good electron acceptors, party because reduction restores...
Text Solution
|
- Quinones are good electron acceptors, party because reduction restores...
Text Solution
|
- Given : Oxidation H(2)O(2) rarr O(2)+2H^(o+)+2e^(-)" "E^(c-)=-0.69V, 2...
Text Solution
|
- The standard reduction potential for the half cell : NO(3)^(c-)(aq)+2H...
Text Solution
|
- Give the decreasing order of stability of the following quinones: a. b...
Text Solution
|
- Assertion (A): Reduction potential value (E^(@)) of o-benzoquine (I...
Text Solution
|
- Which of the following represents o-benzo quinone ?
Text Solution
|
- The standard reduction potential of the half - cell: NO(3)^(-)(aq)+...
Text Solution
|
- The correct stability order of the following three quinones is
Text Solution
|

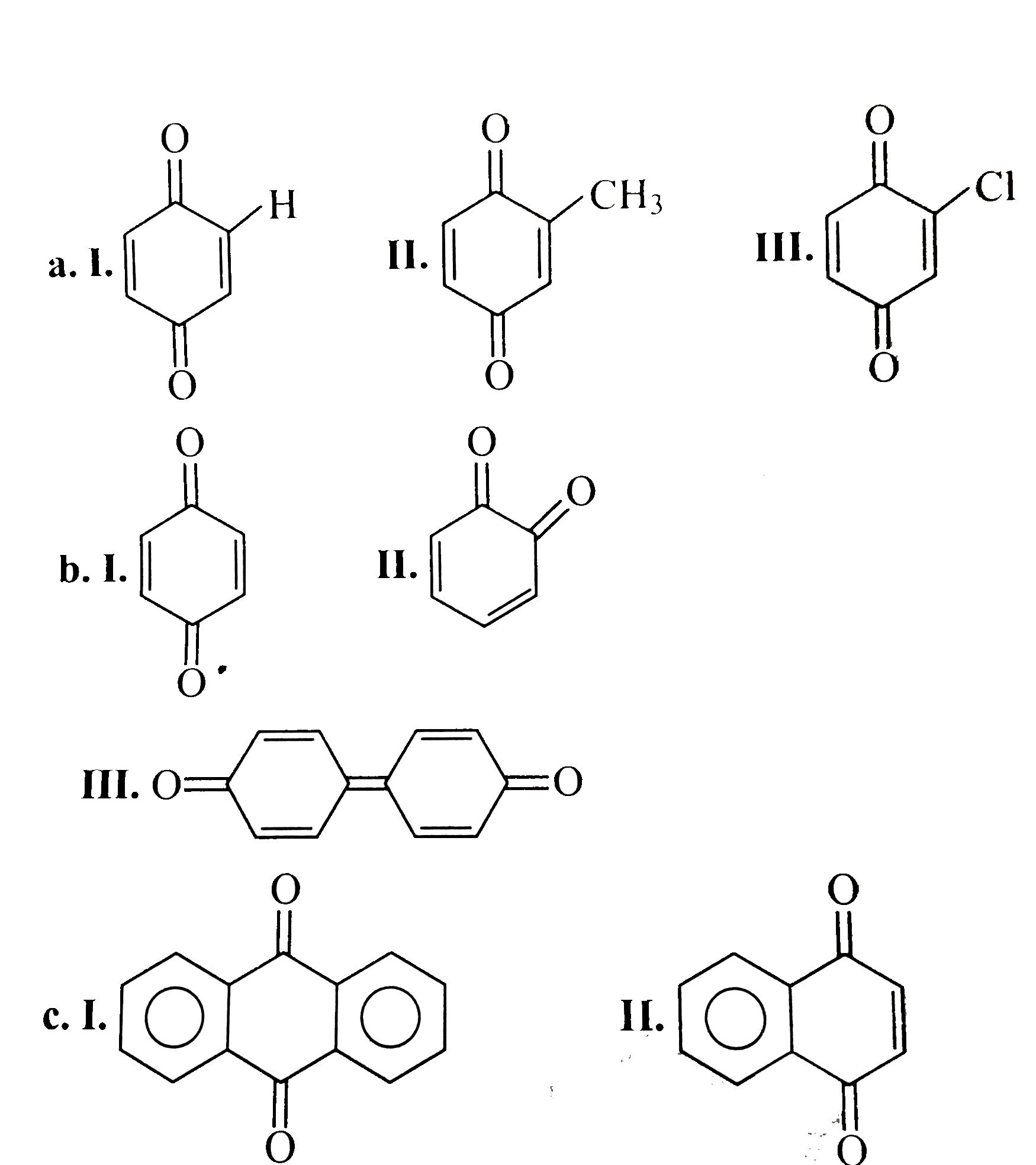
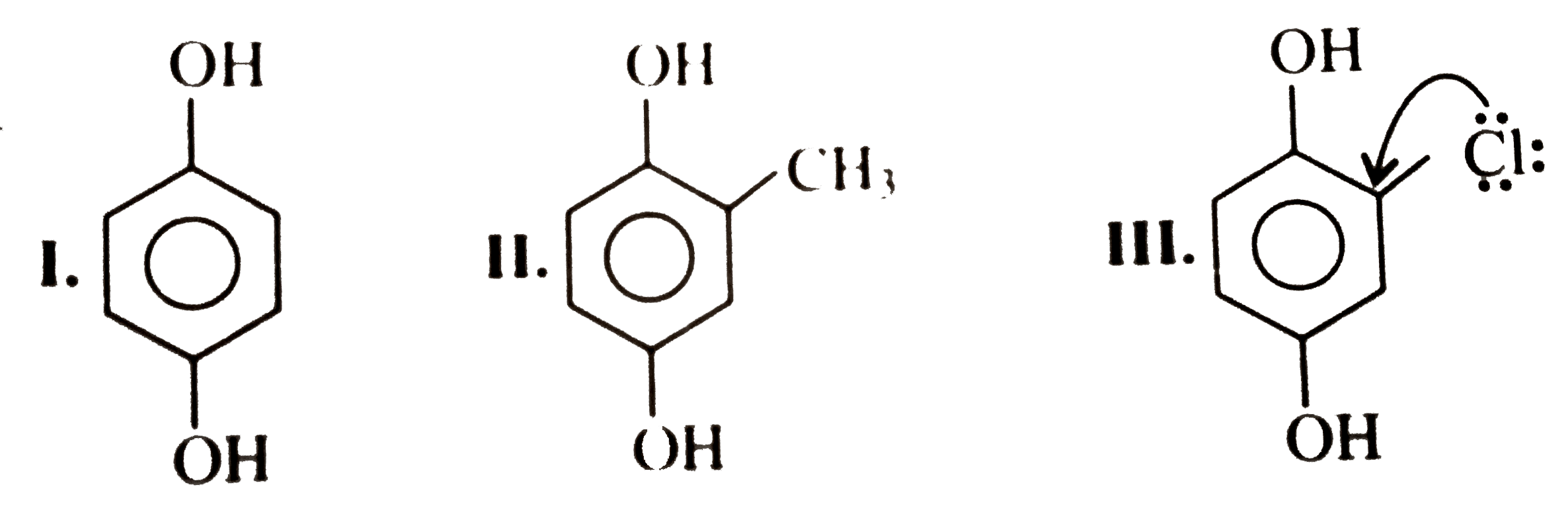
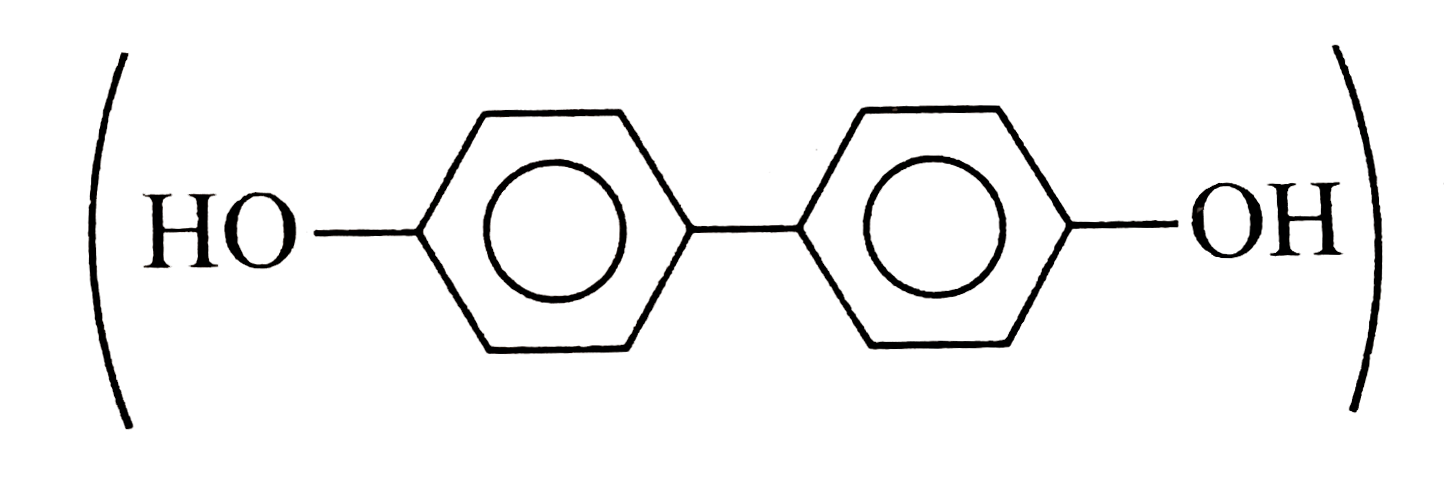 because it generates two aromatic rings which have more resonance energy and greater stability than the reaction of `(I)` and `(II)`, which would generate only one benzene ring.
because it generates two aromatic rings which have more resonance energy and greater stability than the reaction of `(I)` and `(II)`, which would generate only one benzene ring.