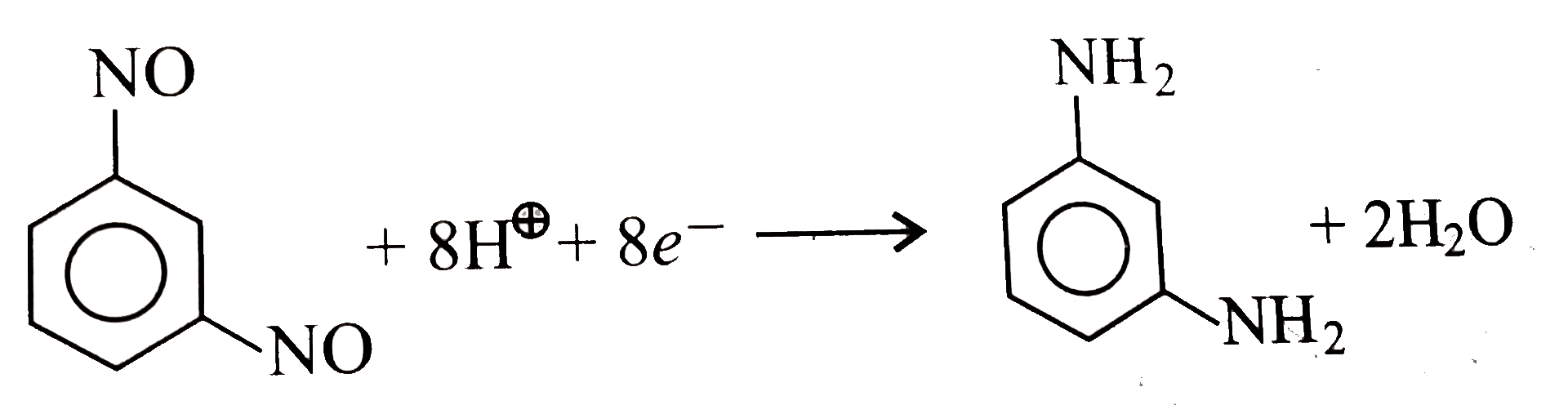Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Aqueous solution of m- dinitroso benzene was electrolyzed for 2 hours ...
Text Solution
|
- Aqueous solution of m- dinitroso benzene was electrolyzed for 2 hors p...
Text Solution
|
- The number of moles of Zn^(2+) ions deposited when a current of 1.5A i...
Text Solution
|
- 1.0 M aqueous solutions of AgNO(3), Cu(NO(3))(2) and Au(NO(3))(3) are ...
Text Solution
|
- Electrolysis of a solution of MnSO(4) in aqueous sulphuric acid is a m...
Text Solution
|
- A current of 0.193 amp is passed through 100 ml of 0.2M NaCl for an ho...
Text Solution
|
- A current of 2A was passed for 1.5 hours through a solution of CuSO(4)...
Text Solution
|
- A current of 2A was passed for 1.5 hours through a solution of CuSO(4)...
Text Solution
|
- A current of 1.5 A is passed through 500 mL of 0.25 M solution of zinc...
Text Solution
|