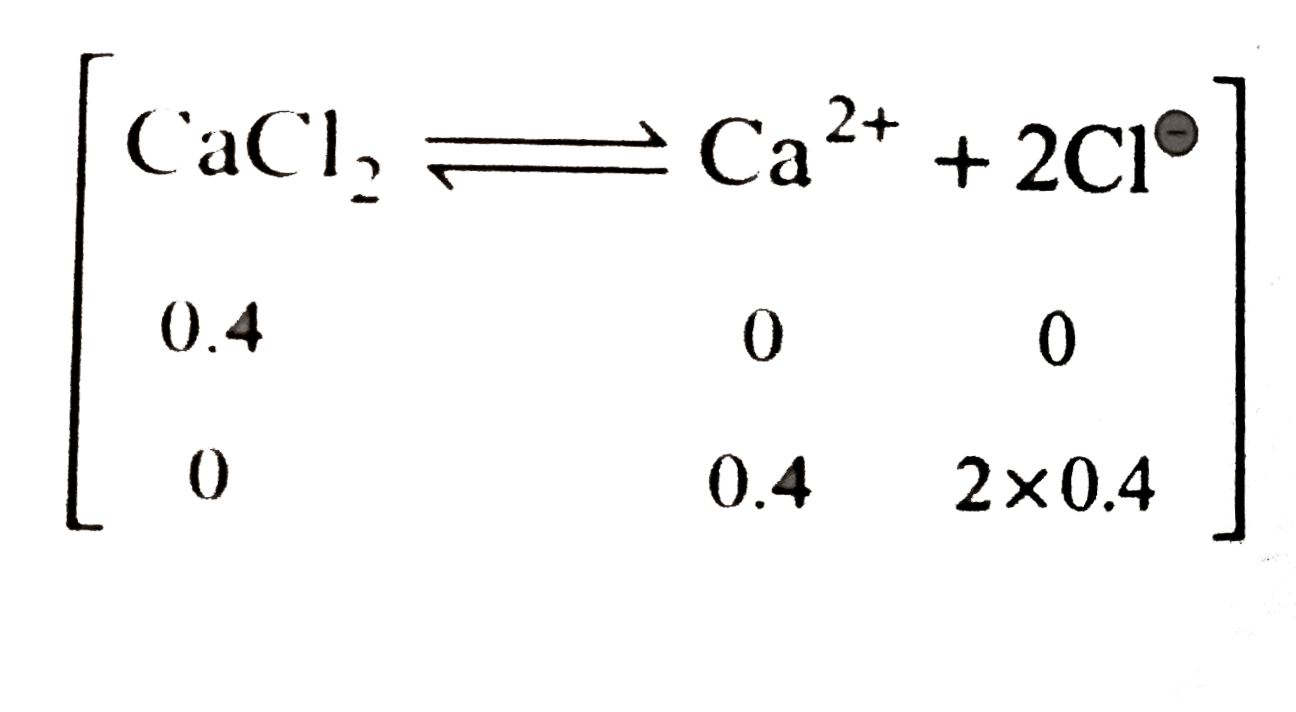A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Ionic strength of 0.4 M CaCl(2) is
Text Solution
|
- Ionic strength of 0.4 M CaCl(2) is
Text Solution
|
- Ionic strength of a solution made by mixing equal volumes of 0.01 M Na...
Text Solution
|
- Calculate the ratio of lattice energies of CaCl(2(s)) and NaCl((s)), i...
Text Solution
|
- The total ionic strength (toal molarity of all ions containing 0.1M "o...
Text Solution
|
- A solution is 0.5M in MgSO(4), 0.1MACl(3) and 0.2 M in (NH(4))(2)SO(4...
Text Solution
|
- Calculate the ionic strength of a solution containing 0.2M NaCL and 0....
Text Solution
|
- Arrange the following in decreasing order of ionic character . CaCl(2)...
Text Solution
|
- Which of the following are not ionic compounds ? (i) CaCl(2) (ii) HCl ...
Text Solution
|