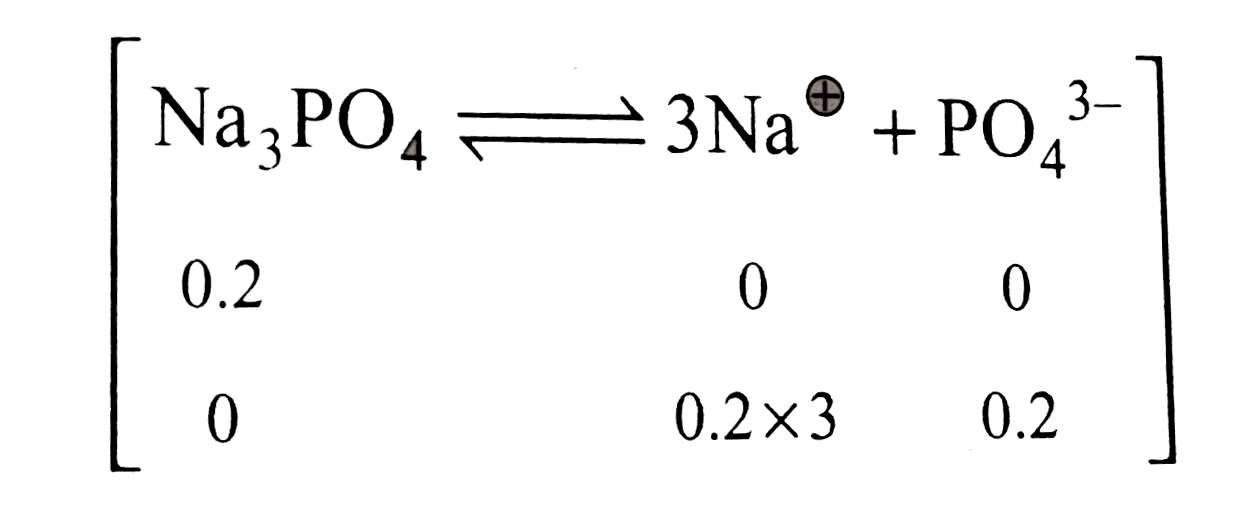
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Ionic strength of 0.4M CaCl(2) is
Text Solution
|
- Ionic strength of 0.4 M CaCl(2) is
Text Solution
|
- Calculate the ratio of lattice energies of CaCl(2(s)) and NaCl((s)), i...
Text Solution
|
- CaCl(2) + C(2)H(5)OH rarr CaCl(2).xC(2) H(5) OH . 'x' is
Text Solution
|
- What is the effect of more electronegative atoms on the strength of an...
Text Solution
|
- Arrange the following in decreasing order of ionic character . CaCl(2)...
Text Solution
|
- Which of the following are not ionic compounds ? (i) CaCl(2) (ii) HCl ...
Text Solution
|
- Between CaCl(2) and KCl, which has a stronger ionic bond? Why?
Text Solution
|
- Compare NaCl and CsCl with respect to ease of formation and also stren...
Text Solution
|