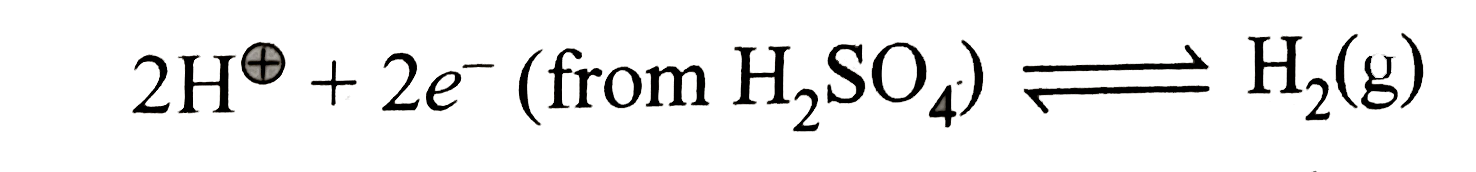A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Marshall's acid (H(2)S(2)O(8)) or peroxodisulphuric acid is prepared b...
Text Solution
|
- Marshall's acid (H(2)S(2)O(8)) or peroxodisulphuric acid is prepared b...
Text Solution
|
- Marshell's acid is prepared by the electrolytic oxidation of H(2)SO(4)...
Text Solution
|
- Out of H(2)S(2)O(3),H(2)S(4)O(6),H(2)SO(5) and H(2)S(2)O(8) peroxy aci...
Text Solution
|
- The oxidation number of sulphur in H(2)SO(4),H(2)S(2)O(4) and H(2)S(2)...
Text Solution
|
- Peroxy disulphuric acid (H(2)S(2)O(8)) can be prepared by electrolytic...
Text Solution
|
- One of the methods of preparation of per disulphuric acid, H(2)S(2)O(8...
Text Solution
|
- During thhe preparation of H(2)S(2)O(8) (per disulphuric acid) O(2) ga...
Text Solution
|
- During an electrolysis of conc. H(2)SO(4) perdisulphuric acid (H(2)S(2...
Text Solution
|