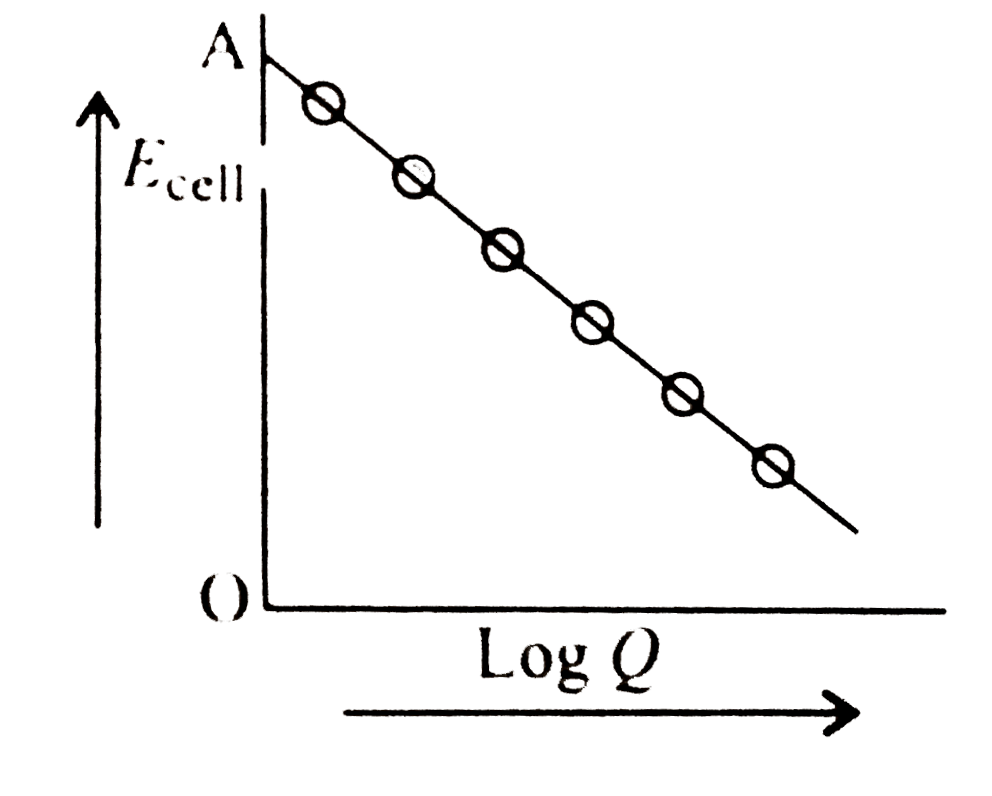
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Zn+Cu^(2+)(aq)hArrCu+Zn^(2+)(aq). Reaction quotient is Q=([Zn^(2+)])...
Text Solution
|
- Zn+Cu^(2+)(aq)hArrCu+Zn^(2+)(aq). Reaction quotient is Q=([Zn^(2+)])/(...
Text Solution
|
- Zn+Cu^(2+)(aq)toCu+Zn^(2+)(aq) . Reaction quotient is Q=([Zn^(2+)])/([...
Text Solution
|
- If in a galvanic cell, the cell reaction is reversed as Cu(s)|Cu^(2...
Text Solution
|
- For the redox reaction : Zn(s)+Cu^(2+)(aq) hArr Zn^(2+)(aq)+Cu(s) Reac...
Text Solution
|
- E^(@) for the cell Zn(s)|Zn^(2+)(aq)|Cu^(2+)(aq)|Cu(s) is 1.1V at 25...
Text Solution
|
- E ^(@) of the cell, zn| Zn^(2+)(aq) ||Cu^(2+) (aq) |Cu is 1.10Vat 25...
Text Solution
|
- Consider the cell reaction Zn+Cu^(2+)(aq)iff Cu + Zn^(2+) (aq). Reacti...
Text Solution
|
- For the following cell reaction Zn+Cu^(2+)(aq) to Zn^(2+) (aq)+Cu E(Zn...
Text Solution
|