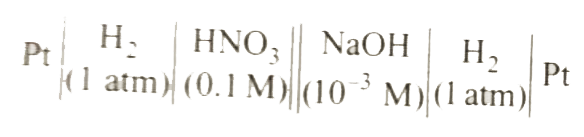A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The potential of the following cell at 25^(@)C is
Text Solution
|
- The standard potential of a cell using the reaction is 1.12. The heat...
Text Solution
|
- The potential of the following cell at 25^(@)C is
Text Solution
|
- Given the following cell at 25^(@)C What will be the potential of the ...
Text Solution
|
- A hydrogen electrode is dipped in a solution at 25^(@)C. The potential...
Text Solution
|
- A huydrgen electrode is dipped is a solution of pH = 3.0 at 25^@C The...
Text Solution
|
- What is the single electrode potential of a half-cell for zinc electro...
Text Solution
|
- The potential of the following cell is 0.34 V at 25^(@)C calculate t...
Text Solution
|
- The hydrogen electrode is dipped in a solution of pH 3 at 25^(@) C. Th...
Text Solution
|