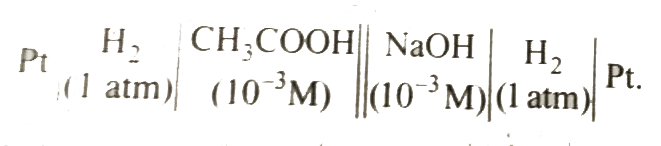A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Given the following cell at 25^(@)C What will be the potential of...
Text Solution
|
- Given the following cell at 25^(@)C What will be the potential of the ...
Text Solution
|
- The potential the cell at 25^(@)C is Given pK(b) of NH(4)OH=4.74
Text Solution
|
- The potential of the cell at 25^(@)C is Given pK(a) of CH(3)COOH and p...
Text Solution
|
- Calculate the pH of the following mixture 50mL of 0.05M CH(3)COOH+50...
Text Solution
|
- Select the indicator form the given table for titratioin of 20 mL of 0...
Text Solution
|
- pK(a) of CH(3)COOH is 4.74 . The pH of 0.01 M CH(3)COONa IS
Text Solution
|
- The ratio of volumes of CH(3)COOH0.1 (N) to CH(3)COONa 0.1 (N) require...
Text Solution
|
- Calculate the buffer capacity of 1L solution of : (i)0.1MCH(3)COOH a...
Text Solution
|