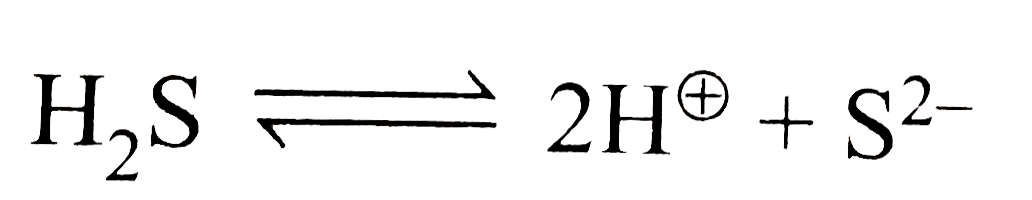A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A half cell reaction Ag(2)S(s)+2e^(-) rarr 3Ag(s)+S^(2-) is carried ou...
Text Solution
|
- A half cell reaction Ag(2)S(s)+2e^(-) rarr 3Ag(s)+S^(2-) is carried ou...
Text Solution
|
- Calculate the half cell potential of a reaction Ag(2)S + 2e rarr 2Ag +...
Text Solution
|
- A button cell used in watched funcations as follwing Zn(s)+Ag(2)O(s)+H...
Text Solution
|
- Calculate the cell potential of a cell having reaction Ag(2)S +2e^(-) ...
Text Solution
|
- To precipitate as Ag(2)S(s) , all Ag^(+) present in 250mL of a saturat...
Text Solution
|
- A button cell used in watches function as following Zn(s)+Ag(2)O(s)+H(...
Text Solution
|
- A button cell used in watches functions as follows: Zn(s)+Ag(2)O(s)+H(...
Text Solution
|
- In the cell reaction Ag((s))+Cu(aq))^(2+)+Br((aq))^(-) to AgBr((s))+...
Text Solution
|