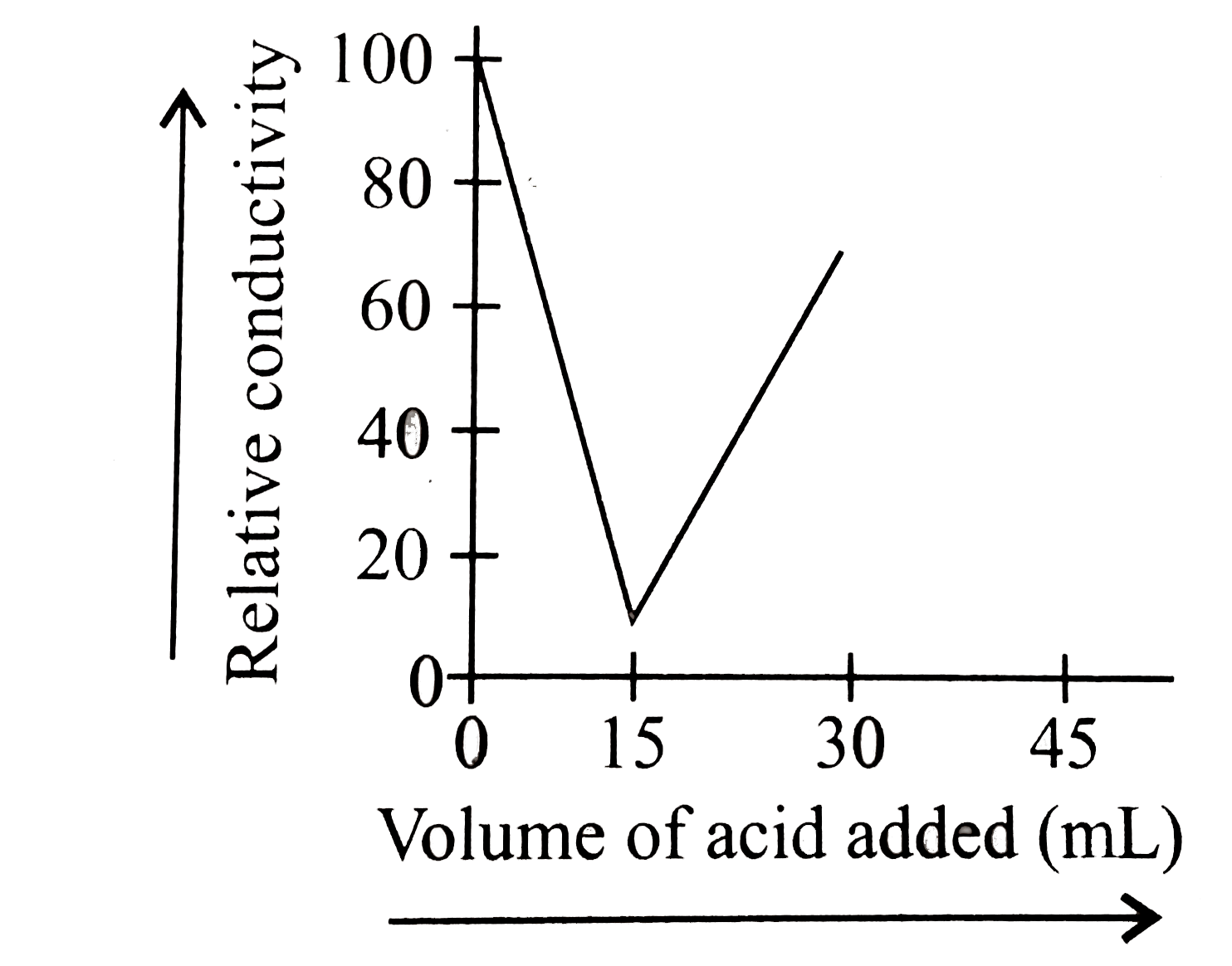
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 20 Ml of KOH solution was titrated with 0.20 M H(2)SO(4) solution in a...
Text Solution
|
- 10 mL solution of H(2)SO(4) and H(2)C(2)O(4) (oxalic acid), on titrati...
Text Solution
|
- 20 Ml of KOH solution was titrated with 0.20 M H(2)SO(4) solution in a...
Text Solution
|
- A 100 ml solution of 0.1 N HCl was titrated with 0.2 ? N NaOH solution...
Text Solution
|
- What volume (in ml) of 0.2 M H(2)SO(4) solution should be mixed with t...
Text Solution
|
- A 20.0 mL sample of a 0.20 M solution of the weak diprotic acid H(2)A ...
Text Solution
|
- The volume of a concentrated H(2)SO(4), mixed with 0.5 N KOH to prepar...
Text Solution
|
- Upon titrating 50mL of 1.96%(w//v) H(2)SO(4) with a KOH solution (cont...
Text Solution
|
- 2 litre of 9.8% w/w H(2)SO(4) (d = 1.5 gm/ml) solution is mixed with 3...
Text Solution
|