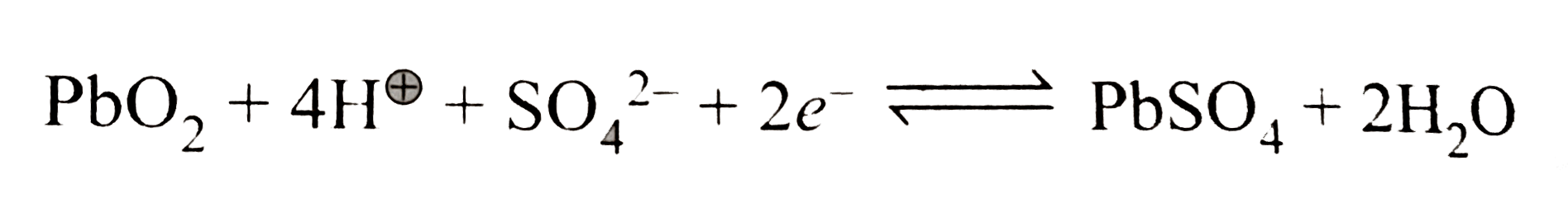Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- During the discharge of a lead storage battery, the density of 40% H(2...
Text Solution
|
- A lead storage cell is discharged which causes H(2)SO(4) electrolyte t...
Text Solution
|
- During the discharge of a lead storage battery, the density of 40% H(2...
Text Solution
|
- The density of H(2)SO(4) ………………………….. in lead storage cell during dis...
Text Solution
|
- Asseration: Equivalent mass of H(2)SO(4) in lead storage battery is 49...
Text Solution
|
- During the discharge of a lead storage battery, the density of sulphur...
Text Solution
|
- During the discharge of a lead storage battery the density of H(2)SO(4...
Text Solution
|
- A lead storage battery consists of a lead anode and a grid of lead pac...
Text Solution
|
- A lead storage battery consists of a lead anode and a grid of lead pac...
Text Solution
|