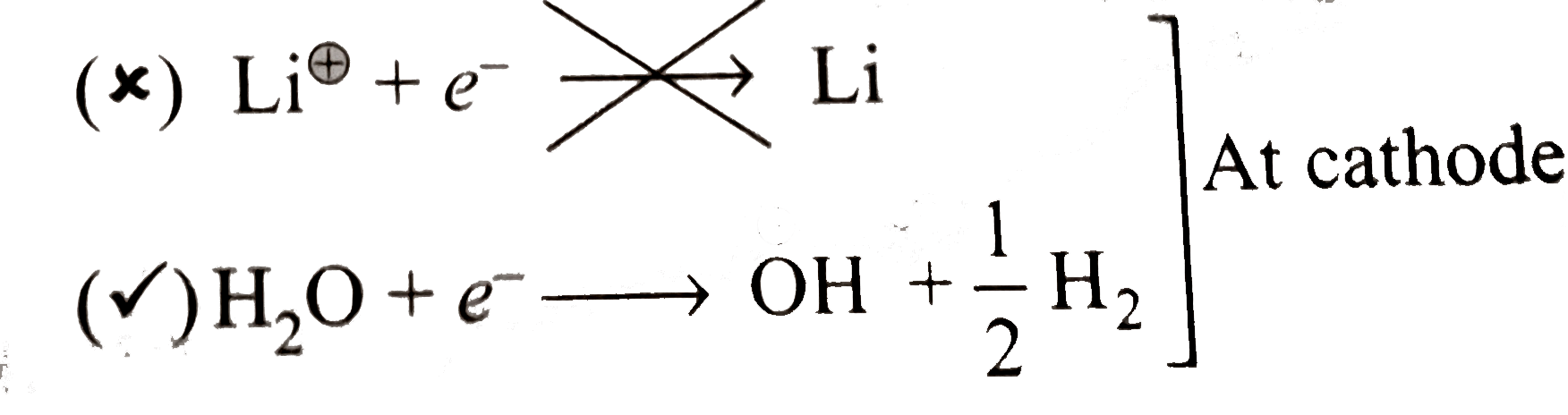Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- When an aqueous solution of LiCl is electrolyzed using graphite electr...
Text Solution
|
- When an aqueous solution of CaCl(2) is electrolyzed using inert electr...
Text Solution
|
- When an aqueous solution of LiCl is electrolyzed using graphite electr...
Text Solution
|
- A dilute aqueous solution of Na(2)SO(4) is electrolyzed using platinum...
Text Solution
|
- सोडियम सल्फेट के जलीय विलयन का विद्युत-अपघटन किया जाता है। कैथोड पर म...
Text Solution
|
- When an aqueous solution of lithium chloride is electrolysed using gra...
Text Solution
|
- When aqueous solution of a calcium salt is electrolyzed, the product a...
Text Solution
|
- when AgNO3 The aqueous solution is electrolyzed between the platinum e...
Text Solution
|
- After the electrolysis of aqueous solution of NaCl using Pt electrodes...
Text Solution
|